A High-Throughput Workflow to Study Remodeling of Extracellular Matrix-Based Microtissues
- PMID: 30430922
- PMCID: PMC6352510
- DOI: 10.1089/ten.TEC.2018.0290
A High-Throughput Workflow to Study Remodeling of Extracellular Matrix-Based Microtissues
Erratum in
-
Correction to: A High-Throughput Workflow to Study Remodeling of Extracellular Matrix-Based Microtissues by Crampton AL, Cummins KA, and Wood DK. Tissue Eng Part C Methods 2019;25(1):25-36. DOI: 10.1089/ten.tec.2018.0290.Tissue Eng Part C Methods. 2019 Jul;25(7):446. doi: 10.1089/ten.tec.2018.0290.correx. Epub 2019 Jun 5. Tissue Eng Part C Methods. 2019. PMID: 31166828 Free PMC article. No abstract available.
Abstract
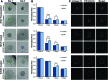
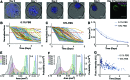
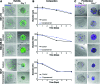
The described microtissue-microwell workflow is uniquely suited for high-throughput study of extracellular matrix (ECM) remodeling at the molecular, cellular, and tissue levels and demonstrates possibilities of studying progressive, heterogeneous diseases in a way that is meaningful for drug discovery and development. We outline several assays that can be utilized in studying tissue-level diseases and functions that involve cell-ECM interactions and ECM remodeling (e.g., cancer, fibrosis, wound healing) in pursuit of an improved three-dimensional cell culturing system. Finally, we demonstrate the ability to cryopreserve cells encapsulated in microtissue constructs while remaining highly viable, proliferative, and retaining cell functions that are involved in ECM remodeling.
Keywords: 3D cell culture; ECM remodeling; droplets; high-throughput screening; microfluidics; microtissues.
Conflict of interest statement
No competing financial interests exist.
Figures



References
-
- Gialeli C., Theocharis A.D., and Karamanos N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 278, 16, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

