Long non-coding RNA HOTAIR mediates the switching of histone H3 lysine 27 acetylation to methylation to promote epithelial-to-mesenchymal transition in gastric cancer
- PMID: 30431069
- PMCID: PMC6254860
- DOI: 10.3892/ijo.2018.4625
Long non-coding RNA HOTAIR mediates the switching of histone H3 lysine 27 acetylation to methylation to promote epithelial-to-mesenchymal transition in gastric cancer
Abstract
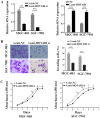
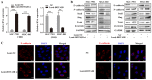
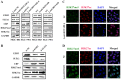
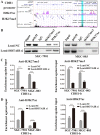
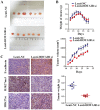
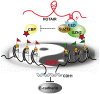
HOX transcript antisense intergenic RNA (HOTAIR), a well‑known long non‑coding RNA, plays an important role in the regulation of epithelial‑to‑mesenchymal transition (EMT). In this study, we propose a novel mechanism through which HOTAIR promotes EMT by switching histone H3 lysine 27 acetylation to methylation at the E‑cadherin promoter, which induces the transcriptional inhibition of E‑cadherin. HOTAIR recruits polycomb repressive complex 2 (PRC2) to catalyze H3K27me3; however, whether HOTAIR is associated with the acetylation of histone H3 lysine 27, a marker of transcriptional activation, and the mechanisms through which HOTAIR triggers the metastasis of gastric cancer (GC) by epigenetic regulation remain largely unknown. In this study, HOTAIR knockdown significantly reversed EMT by increasing the expression of E‑cadherin in GC cells. Additionally, the loss of PRC2 activity induced by HOTAIR knockdown resulted in a global decrease in H3K27 methylation and an increase in H3K27 acetylation. Furthermore, HOTAIR recruits PRC2 (which consists of H3K27 methyltransferase EZH2, SUZ12 and EED), which may inhibit the reaction between the acetyltransferase CBP and H3K27 acetylation. On the whole, the findings of this study suggested that the HOTAIR‑mediated acetylation to methylation switch was associated with the transcriptional inhibition of E‑cadherin. HOTAIR can promote the development of GC through the epigenetic regulation of E‑cadherin, switching the state of the E‑cadherin promoter from the transcriptionally active to the transcriptionally repressive state.
Keywords: HOTAIR; gastric cancer; histone modification; epithelial-to-mesenchymal transition; molecular biomarker.
Figures

References
-
- Yan Y, Zhang J, Li JH, Liu X, Wang JZ, Qu HY, Wang JS, Duan XY. High tumor-associated macrophages infiltration is associated with poor prognosis and may contribute to the phenomenon of epithelial-mesenchymal transition in gastric cancer. Onco Targets Ther. 2016;9:3975–3983. doi: 10.2147/OTT.S103112. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

