c‑Jun N‑terminal kinase inhibition attenuates early brain injury induced neuronal apoptosis via decreasing p53 phosphorylation and mitochondrial apoptotic pathway activation in subarachnoid hemorrhage rats
- PMID: 30431087
- PMCID: PMC6297759
- DOI: 10.3892/mmr.2018.9640
c‑Jun N‑terminal kinase inhibition attenuates early brain injury induced neuronal apoptosis via decreasing p53 phosphorylation and mitochondrial apoptotic pathway activation in subarachnoid hemorrhage rats
Abstract
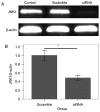
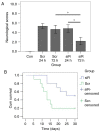
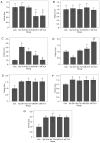
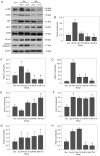
Early brain injury (EBI)‑induced neuronal apoptosis is primarily responsible for the subsequent complications of aneurysmal subarachnoid hemorrhage (aSAH), which may increase the risk of mortality in patients with aSAH. c‑Jun N‑terminal kinase (JNK) has been demonstrated to be a promoter of EBI‑induced cell apoptosis, although the mechanism has yet to be fully elucidated. The present study aimed to explore whether the role of JNK1 is associated with tumor protein p53 (p53), which is one of the most important factor that triggers cell apoptosis. JNK1 expression was downregulated via in vivo small interfering RNA transfection in an aSAH rat model in order to assess differences in the behavior, survival times, morphology and genetics of the experimental animals. The results revealed that JNK1 inhibition improved the neurological scores and survival times of SAH rats by interrupting cascaded neuronal apoptosis. The interruption of EBI‑induced neuronal apoptosis may originate from a decrease in the level of p53 phosphorylation and deactivation of the downstream mitochondrial apoptotic pathway. Taken together, these results suggest that JNK1 may be a promising target for improving the prognosis of patients with aSAH.
Keywords: aneurysmal subarachnoid hemorrhage; early brain injury; apoptosis; c-Jun N-terminal kinase; tumor protein 53.
Figures



Similar articles
-
The Ras/Raf/Erk Pathway Mediates the Subarachnoid Hemorrhage-Induced Apoptosis of Hippocampal Neurons Through Phosphorylation of p53.Mol Neurobiol. 2016 Oct;53(8):5737-48. doi: 10.1007/s12035-015-9490-x. Epub 2015 Oct 26. Mol Neurobiol. 2016. PMID: 26497030
-
Apolipoprotein E Deficiency Aggravates Neuronal Injury by Enhancing Neuroinflammation via the JNK/c-Jun Pathway in the Early Phase of Experimental Subarachnoid Hemorrhage in Mice.Oxid Med Cell Longev. 2019 Dec 26;2019:3832648. doi: 10.1155/2019/3832648. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31949876 Free PMC article.
-
DLK silencing attenuated neuron apoptosis through JIP3/MA2K7/JNK pathway in early brain injury after SAH in rats.Neurobiol Dis. 2017 Jul;103:133-143. doi: 10.1016/j.nbd.2017.04.006. Epub 2017 Apr 8. Neurobiol Dis. 2017. PMID: 28396258 Free PMC article.
-
Recombinant OX40 attenuates neuronal apoptosis through OX40-OX40L/PI3K/AKT signaling pathway following subarachnoid hemorrhage in rats.Exp Neurol. 2020 Apr;326:113179. doi: 10.1016/j.expneurol.2020.113179. Epub 2020 Jan 10. Exp Neurol. 2020. PMID: 31930990 Review.
-
Early brain injury following aneurysmal subarachnoid hemorrhage: emphasis on cellular apoptosis.Turk Neurosurg. 2012;22(5):529-33. doi: 10.5137/1019-5149.JTN.5731-12.1. Turk Neurosurg. 2012. PMID: 23015327 Review.
Cited by
-
New Mechanisms and Targets of Subarachnoid Hemorrhage: A Focus on Mitochondria.Curr Neuropharmacol. 2022;20(7):1278-1296. doi: 10.2174/1570159X19666211101103646. Curr Neuropharmacol. 2022. PMID: 34720082 Free PMC article. Review.
-
The mechanism and relevant mediators associated with neuronal apoptosis and potential therapeutic targets in subarachnoid hemorrhage.Neural Regen Res. 2023 Feb;18(2):244-252. doi: 10.4103/1673-5374.346542. Neural Regen Res. 2023. PMID: 35900398 Free PMC article. Review.
-
Oxidative Stress and Intracranial Hypertension after Aneurysmal Subarachnoid Hemorrhage.Antioxidants (Basel). 2022 Dec 8;11(12):2423. doi: 10.3390/antiox11122423. Antioxidants (Basel). 2022. PMID: 36552631 Free PMC article. Review.
-
The blood-brain barrier and the neurovascular unit in subarachnoid hemorrhage: molecular events and potential treatments.Fluids Barriers CNS. 2022 Apr 11;19(1):29. doi: 10.1186/s12987-022-00312-4. Fluids Barriers CNS. 2022. PMID: 35410231 Free PMC article. Review.
-
Wnt Pathway: An Emerging Player in Vascular and Traumatic Mediated Brain Injuries.Front Physiol. 2020 Sep 18;11:565667. doi: 10.3389/fphys.2020.565667. eCollection 2020. Front Physiol. 2020. PMID: 33071819 Free PMC article. Review.
References
-
- Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: A randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2) Lancet Neurol. 2011;10:618–625. doi: 10.1016/S1474-4422(11)70108-9. - DOI - PubMed
-
- Yuksel S, Tosun YB, Cahill J, Solaroglu I. Early brain injury following aneurysmal subarachnoid hemorrhage: Emphasis on cellular apoptosis. Turk Neurosurg. 2012;22:529–533. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

