A novel role for programmed cell death receptor ligand 2 in sepsis-induced hepatic dysfunction
- PMID: 30431333
- PMCID: PMC6383374
- DOI: 10.1152/ajpgi.00204.2018
A novel role for programmed cell death receptor ligand 2 in sepsis-induced hepatic dysfunction
Abstract
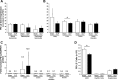
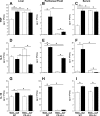
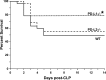
The liver is an organ that, when dysfunctional in a septic patient, is strongly associated with morbidity and mortality. Understanding the pathophysiology of liver failure during sepsis may lead to improved diagnostics and potential therapeutic targets. Historically, programmed cell death receptor (PD) ligand 1 (PD-L1) has been considered the primary ligand for its checkpoint molecule counterpart, PD-1, with PD-L2 rarely in the immunopathological spotlight. PD-1 and PD-L1 contribute to liver dysfunction in a murine cecal ligation and puncture (CLP) model of sepsis, but virtually nothing is known about PD-L2's role in sepsis. Therefore, our central hypothesis was that sepsis-induced changes in hepatic PD-L2 expression contributed to worsened liver function and, subsequently, more pronounced morbidity and mortality. We found that although PD-L1 gene deficiency attenuated the hepatic dysfunction seen in wild-type mice after CLP, the loss of PD-L2 appeared to actually worsen indices of liver function along with a trend toward higher liver tissue vascular permeability. Conversely, some protective effects of PD-L2 gene deletion were noted, such as reduced liver/peritoneal bacterial load and reduced IL-6, IL-10, and macrophage inflammatory protein 2 levels following CLP. These diverse actions, as well as the unique expression pattern of PD-L2, may explain why no overt survival advantage could be witnessed in the septic PD-L2-/- mice. Taken together, these data suggest that although PD-L2 has some selective effects on the hepatic response seen in the septic mouse, these factors are not sufficient to alter septic mortality in this adult murine model. NEW & NOTEWORTHY Our study shows not only that ligands of the checkpoint protein PD-1 respond inversely to a stressor such as septic challenge (PD-L2 declines, whereas PD-L1 rises) but also that aspects of liver dysfunction increase in septic mice lacking the PD-L2 gene. Furthermore, these differences in PD-L2 gene-deficient animals culminated in the abrogation of the survival advantage seen in the septic PD-L1-knockout mice, suggesting that PD-L2 may have roles beyond a simple immune tolerogen.
Keywords: inflammation; posttranslational modification; programmed cell death receptor ligand 1; programmed cell death receptor-1; sepsis.
Figures



Similar articles
-
Contribution of programmed cell death receptor (PD)-1 to Kupffer cell dysfunction in murine polymicrobial sepsis.Am J Physiol Gastrointest Liver Physiol. 2016 Aug 1;311(2):G237-45. doi: 10.1152/ajpgi.00371.2015. Epub 2016 Jun 10. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27288425 Free PMC article.
-
Glutamine Administration in Early or Late Septic Phase Downregulates Lymphocyte PD-1/PD-L1 Expression and the Inflammatory Response in Mice With Polymicrobial Sepsis.JPEN J Parenter Enteral Nutr. 2018 Mar;42(3):538-549. doi: 10.1177/0148607117695245. Epub 2017 Dec 12. JPEN J Parenter Enteral Nutr. 2018. PMID: 28633555
-
Farnesyltransferase inhibitor FTI-277 inhibits PD-L1 expression on septic spleen lymphocytes and promotes spleen lymphocyte activation.Clin Exp Immunol. 2017 Oct;190(1):8-18. doi: 10.1111/cei.12995. Epub 2017 Jul 14. Clin Exp Immunol. 2017. PMID: 28556912 Free PMC article.
-
PD-1 signaling pathway in sepsis: Does it have a future?Clin Immunol. 2021 Aug;229:108742. doi: 10.1016/j.clim.2021.108742. Epub 2021 Apr 24. Clin Immunol. 2021. PMID: 33905818 Review.
-
Cellular and molecular regulation of the programmed death-1/programmed death ligand system and its role in multiple sclerosis and other autoimmune diseases.J Autoimmun. 2021 Sep;123:102702. doi: 10.1016/j.jaut.2021.102702. Epub 2021 Jul 23. J Autoimmun. 2021. PMID: 34311143 Review.
Cited by
-
Insights into sepsis-induced apoptosis: Interplay between programmed cell death and interleukin-7.J Crit Care Med (Targu Mures). 2025 Apr 30;11(2):132-139. doi: 10.2478/jccm-2025-0003. eCollection 2025 Apr. J Crit Care Med (Targu Mures). 2025. PMID: 40386708 Free PMC article. Review.
-
Lymphocyte HVEM/BTLA co-expression after critical illness demonstrates severity indiscriminate upregulation, impacting critical illness-induced immunosuppression.Front Med (Lausanne). 2023 May 25;10:1176602. doi: 10.3389/fmed.2023.1176602. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37305124 Free PMC article.
-
Protective effect of exercise on animals with sepsis: a systematic review of the existing literature.BMC Infect Dis. 2025 Feb 8;25(1):195. doi: 10.1186/s12879-025-10557-7. BMC Infect Dis. 2025. PMID: 39923007 Free PMC article.
-
The Pathogenesis of Sepsis and Potential Therapeutic Targets.Int J Mol Sci. 2019 Oct 29;20(21):5376. doi: 10.3390/ijms20215376. Int J Mol Sci. 2019. PMID: 31671729 Free PMC article. Review.
-
The Effect of Secreted IL-10 from Mesenchymal Stem Cell on Immune Checkpoint Molecules.Acta Inform Med. 2023;31(3):172-175. doi: 10.5455/aim.2023.31.172-175. Acta Inform Med. 2023. PMID: 37781487 Free PMC article.
References
-
- Ayala A, Perrin MM, Kisala JM, Ertel W, Chaudry IH. Polymicrobial sepsis selectively activates peritoneal but not alveolar macrophages to release inflammatory mediators (interleukins-1 and -6 and tumor necrosis factor). Circ Shock 36: 191–199, 1992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

