Airway Mucin Secretion
- PMID: 30431339
- PMCID: PMC6322024
- DOI: 10.1513/AnnalsATS.201806-371AW
Airway Mucin Secretion
Abstract
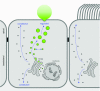
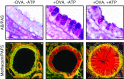
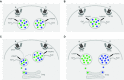
Exocytosis of secreted mucins is the final step in their intracellular processing, resulting in their release into the airway lumen to interact with water and ions to form mucus. Mucins are secreted at a low baseline rate and a high stimulated rate, and both rates are regulated by second messengers acting on components of the exocytic machinery. The principal physiologic function of the low baseline rate is to support steady-state mucociliary clearance of inhaled particles and pathogens that enter the airways during normal breathing. Even in the setting of mucin hyperproduction, baseline secretion generally does not induce mucus occlusion. The principal physiologic function of the high stimulated rate of secretion from both submucosal glands and surface goblet cells in proximal airways appears to be to sweep away larger particles, whereas in distal airways it appears to act in concert with mucin hyperproduction to induce mucus occlusion to trap migrating helminths. Pathophysiologically, stimulated mucin secretion in the setting of mucin hyperproduction from allergic or other types of airway inflammation in the absence of helminth infection causes airflow obstruction and infection. Molecular components of the mucin exocytic machinery are increasingly being identified, and surprisingly, many components are not shared between baseline and stimulated machines. The physiologic significance of the presence of two distinct molecular machines is not yet known, such as whether these interact selectively with secretory granules of different sizes or contents. A full understanding of the mechanism and regulation of airway mucin secretion will provide further insight into pathophysiologic processes and may identify therapeutic strategies to alleviate obstructive airway diseases.
Keywords: exocytosis; mucin; mucus; secretion.
Figures

References
-
- Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

