Intracellular Processing of Human Secreted Polymeric Airway Mucins
- PMID: 30431345
- PMCID: PMC6276985
- DOI: 10.1513/AnnalsATS.201802-143AW
Intracellular Processing of Human Secreted Polymeric Airway Mucins
Abstract
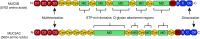
Mucociliary clearance is a crucial component of innate defense of the lung. In respiratory diseases, such as asthma, chronic obstructive pulmonary disease, and cystic fibrosis, mucus with abnormal properties contributes to obstruction of the airways. The failure in function of mucus in airway clearance and pathogen protection leads to chronic infection and risk of death. Polymeric mucins (MUC5AC and MUC5B) provide the structural framework of the airway mucus gel. The intracellular synthesis and assembly of these enormous, polymeric O-linked glycoproteins is a complex, multistage process involving intra- and intermolecular disulfide bond formation and extensive addition of O-glycan chains. The fully formed polymers are packaged in a highly organized and condensed form within secretory granules inside specialized secretory cells, and after the appropriate stimulus, mucins are released and expand to form mucus. This short article brings together the current knowledge on the different steps in the production of mucin polymers and the molecular mechanisms that condense them into a packaged form in secretory granules. It is by unraveling the molecular mechanisms that control intracellular mucin supramolecular structure that we might gain new insight into what determines mucus gel properties in health and disease.
Keywords: MUC5AC; MUC5B; mucin; mucus.
Figures
Similar articles
-
Airway Mucin Secretion.Ann Am Thorac Soc. 2018 Nov;15(Suppl 3):S164-S170. doi: 10.1513/AnnalsATS.201806-371AW. Ann Am Thorac Soc. 2018. PMID: 30431339 Free PMC article. Review.
-
Assembly, Release, and Transport of Airway Mucins in Pigs and Humans.Ann Am Thorac Soc. 2018 Nov;15(Suppl 3):S159-S163. doi: 10.1513/AnnalsATS.201804-238AW. Ann Am Thorac Soc. 2018. PMID: 30431338 Free PMC article. Review.
-
Mucins, Mucus, and Goblet Cells.Chest. 2018 Jul;154(1):169-176. doi: 10.1016/j.chest.2017.11.008. Epub 2017 Nov 21. Chest. 2018. PMID: 29170036 Review.
-
Mucins MUC5AC and MUC5B Are Variably Packaged in the Same and in Separate Secretory Granules.Am J Respir Crit Care Med. 2022 Nov 1;206(9):1081-1095. doi: 10.1164/rccm.202202-0309OC. Am J Respir Crit Care Med. 2022. PMID: 35776514 Free PMC article.
-
Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B.Biochem J. 2002 Feb 1;361(Pt 3):537-46. doi: 10.1042/0264-6021:3610537. Biochem J. 2002. PMID: 11802783 Free PMC article.
Cited by
-
Assembly Mechanism of Mucin and von Willebrand Factor Polymers.Cell. 2020 Oct 29;183(3):717-729.e16. doi: 10.1016/j.cell.2020.09.021. Epub 2020 Oct 7. Cell. 2020. PMID: 33031746 Free PMC article.
-
Physiology and pathophysiology of human airway mucus.Physiol Rev. 2022 Oct 1;102(4):1757-1836. doi: 10.1152/physrev.00004.2021. Epub 2022 Jan 10. Physiol Rev. 2022. PMID: 35001665 Free PMC article. Review.
-
CFTR dysfunction leads to defective bacterial eradication on cystic fibrosis airways.Front Physiol. 2024 Apr 18;15:1385661. doi: 10.3389/fphys.2024.1385661. eCollection 2024. Front Physiol. 2024. PMID: 38699141 Free PMC article. Review.
-
Lack of airway submucosal glands impairs respiratory host defenses.Elife. 2020 Oct 7;9:e59653. doi: 10.7554/eLife.59653. Elife. 2020. PMID: 33026343 Free PMC article.
-
Chair's Summary: Secreted Mucins in Lung Diseases.Ann Am Thorac Soc. 2018 Nov;15(Suppl 3):S140-S142. doi: 10.1513/AnnalsATS.201810-666AW. Ann Am Thorac Soc. 2018. PMID: 30431341 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

