Host and Viral Determinants of Respiratory Syncytial Virus-induced Airway Mucus
- PMID: 30431348
- PMCID: PMC6322028
- DOI: 10.1513/AnnalsATS.201806-380AW
Host and Viral Determinants of Respiratory Syncytial Virus-induced Airway Mucus
Abstract
Respiratory syncytial virus (RSV) is a leading cause of hospitalization of infants worldwide each year. Both host and viral factors host factors predispose a subset of what appear to be healthy infants to severe RSV-induced disease. In this review, we outline many genetic and immunologic factors that contribute to airway obstruction that contributes to the severity of RSV infection.
Keywords: IL-13; RSV; interferon; mucus.
Figures

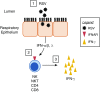
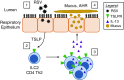
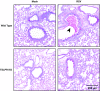
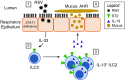

References
-
- Fryzek JP, Martone WJ, Groothuis JR. Trends in chronologic age and infant respiratory syncytial virus hospitalization: an 8-year cohort study. Adv Ther. 2011;28:195–201. - PubMed
-
- Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J. 2002;21:629–632. - PubMed
-
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5) Suppl:S127–S132. - PubMed
-
- Glick AF, Kjelleren S, Hofstetter AM, Subramony A. RSV hospitalizations in comparison with regional RSV activity and inpatient palivizumab administration, 2010–2013. Hosp Pediatr. 2017;7:271–278. - PubMed
-
- Berger TM, Aebi C, Duppenthaler A, Stocker M Swiss Pediatric Surveillance Unit. Prospective population-based study of RSV-related intermediate care and intensive care unit admissions in Switzerland over a 4-year period (2001–2005) Infection. 2009;37:109–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

