Efficacy and safety of rituximab in childhood-onset, difficult-to-treat nephrotic syndrome: A multicenter open-label trial in Korea
- PMID: 30431588
- PMCID: PMC6257685
- DOI: 10.1097/MD.0000000000013157
Efficacy and safety of rituximab in childhood-onset, difficult-to-treat nephrotic syndrome: A multicenter open-label trial in Korea
Abstract
Background: The anti-CD20 monoclonal antibody rituximab (RTX) has been proposed as a rescue therapy for difficult-to-treat nephrotic syndrome (NS). We conducted a clinical trial to evaluate the efficacy and safety of RTX in children with difficult-to-treat NS dependent on or resistant to steroids and calcineurin inhibitors (CNIs).
Methods: A multicenter open-label trial was performed at 8 major pediatric nephrology centers in Korea. The investigation consisted of a randomized controlled trial for steroid- and CNI-dependent NS (DDNS; randomization into the RTX group and the control group, at a ratio of 2:1) and a single-arm study of steroid and CNI-resistant NS (DRNS). DDNS patients in the RTX group and DRNS patients received a single dose of intravenous RTX (375 mg/m of body surface area) for B-cell depletion. A second RTX dose was administered at week 2 if the first dose failed to achieve depletion of CD19(+) cells. The primary endpoint was rate of maintaining remission at 6 months after treatment for DDNS and rate of remission achievement for DRNS.
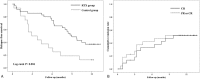
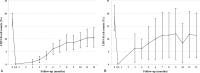
Results: Sixty-one children with DDNS were enrolled while in remission and randomized to the control group (21 patients) or the RTX group (40 patients). At 6 months after treatment, the remission rates were 74.3% in the RTX group and 31.3% in the control group (P = .003). The mean duration of remission maintenance was significantly higher in the RTX group than in the control group (9.0 vs 2.9 months, P = .004). Of the 23 patients with DRNS enrolled in the single-arm study and treated with RTX, 9 (39.1%) achieved partial or complete remission within 6 months. Depletion of B cells occurred in all patients with RTX therapy. Thirty patients (50.8% of 59 patients analyzed) experienced mild and transient infusion reaction during RTX administration, and most adverse events were mild.
Conclusions: RTX administration was safe and effective in patients with difficult-to-treat NS. One or 2 doses of RTX may be sufficient to deplete B cells and achieve better control of pediatric NS.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2012;2:139–274.
-
- El-Husseini A, El-Basuony F, Mahmoud I, et al. Long-term effects of cyclosporine in children with idiopathic nephrotic syndrome: a single-centre experience. Nephrol Dial Transplant 2005;20:2433–8. - PubMed
-
- Foster BJ, Shults J, Zemel BS, et al. Interactions between growth and body composition in children treated with high-dose chronic glucocorticoids. Am J Clin Nutr 2004;80:1334–41. - PubMed
-
- Iijima K, Hamahira K, Tanaka R, et al. Risk factors for cyclosporine-induced tubulointerstitial lesions in children with minimal change nephrotic syndrome. Kidney Int 2002;61:1801–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

