Engineering a Coenzyme A Detour To Expand the Product Scope and Enhance the Selectivity of the Ehrlich Pathway
- PMID: 30433765
- PMCID: PMC7195871
- DOI: 10.1021/acssynbio.8b00358
Engineering a Coenzyme A Detour To Expand the Product Scope and Enhance the Selectivity of the Ehrlich Pathway
Abstract
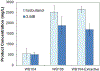
The Ehrlich pathway is a major route for the renewable production of higher alcohols. However, the product scope of the Ehrlich pathway is restricted, and the product selectivity is suboptimal. Here, we demonstrate that a Coenzyme A (CoA) detour, which involves conversion of the 2-keto acids into acyl-CoAs, expands the biological toolkit of reaction chemistries available in the Ehrlich pathway to include the gamut of CoA-dependent enzymes. As a proof-of-concept, we demonstrated the first biosynthesis of a tertiary branched-alcohol, pivalcohol, at a level of ∼10 mg/L from glucose in Escherichia coli, using a pivalyl-CoA mutase from Xanthobacter autotrophicus. Furthermore, engineering an enzyme in the CoA detour, the Lactobacillus brevis CoA-acylating aldehyde dehydrogenase, allowed stringent product selectivity. Targeted production of 3-methyl-1-butanol (3-MB) in E. coli mediated by the CoA detour showed a 3-MB:side-product (isobutanol) ratio of >20, an increase over the ratios previously achieved using the conventional Ehrlich pathway.
Keywords: Ehrlich pathway; higher alcohol; metabolic engineering; pivalyl-CoA mutase; tertiary branched-chemicals.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

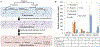

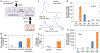
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

