L-DOPA-Induced Motor Impairment and Overexpression of Corticostriatal Synaptic Components Are Improved by the mGluR5 Antagonist MPEP in 6-OHDA-Lesioned Rats
- PMID: 30439288
- PMCID: PMC6238196
- DOI: 10.1177/1759091418811021
L-DOPA-Induced Motor Impairment and Overexpression of Corticostriatal Synaptic Components Are Improved by the mGluR5 Antagonist MPEP in 6-OHDA-Lesioned Rats
Abstract
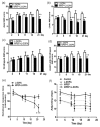
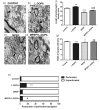
Levodopa (L-DOPA) is still the most effective drug for the treatment of Parkinson's disease (PD). However, the long-term therapy often triggers L-DOPA-induced dyskinesia (LID). Metabotropic glutamate receptor type 5 (mGluR5) is abundant in the basal ganglia, and its inhibition is thought to modulate postsynaptic excitatory synaptic transmission and glutamate hyperactivity in PD and LID. In this report, we examined the effects of mGluR5-specific antagonist 2-methyl-6-(phenylethynyl)pyridine (MPEP) on LID and synaptic components in the PD model rat. We found the selective mGluR5 antagonist MPEP attenuated abnormal involuntary movements, prolonged the duration of rotational response, reversed the decrease of left forepaw adjusting steps, and reduced overexpression of striatal mGluR5 in the LID rats. Moreover, our results showed much thicker postsynaptic densities, narrower synapse cleft, as well as the increased ratio of perforated synapses induced by L-DOPA treatment, while coadministration of L-DOPA and MPEP reversed these postsynaptic effects. Finally, MPEP reduced overexpression of the two postsynaptic proteins (PSD-95 and SAP102) induced by L-DOPA treatment. Hence, these results provide evidence that aberrant neural plasticity at corticostriatal synapses in the striatum is closely correlated with the occurrence of LID, and targeted inhibition of mGluR5 by MPEP alleviates LID in the PD rat model.
Keywords: L-DOPA-induced dyskinesia; MPEP; Parkinson’s disease; mGluR5; postsynaptic densities.
Figures

References
-
- Addex Therapeutics, Geneva, Switzerland (Media Release). (2012, March). Retrieved from http://www.addextherapeutics.com/investors/pressreleases/newsdetails/art... (accessed 11.02.13.).
-
- Ahlskog J., Muenter M. (2001). Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord, 16, 448–458. - PubMed
-
- Bastide M. F., et al. (2015). Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson's disease. Prog Neurobiol, 132, 96–168. - PubMed
-
- Blandini F., Armentero M. T. (2012). New pharmacological avenues for the treatment of L-DOPA-induced dyskinesias in Parkinson's disease: Targeting glutamate and adenosine receptors. Expert Opin Investig Drugs, 21(2), 153–168. - PubMed
-
- Calon F., Rajput A. H., Hornykiewicz O., Bedard P. J., Di Paolo T. (2003). Levodopa induced motor complications are associated with alterations of glutamate receptors in Parkinson’s disease. Neurobiol Dis, 14, 404–416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

