Satisfactory Response With Achieving Maintenance Low-Dose Prednisone in Generalized Myasthenia Gravis
- PMID: 30439750
- PMCID: PMC6241299
- DOI: 10.1097/CND.0000000000000219
Satisfactory Response With Achieving Maintenance Low-Dose Prednisone in Generalized Myasthenia Gravis
Abstract
Objectives: To estimate the satisfactory response rate (SR%) with achieving maintenance, low-dose prednisone in acetylcholine receptor antibody-positive generalized myasthenia gravis.
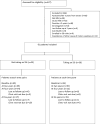
Methods: In this retrospective study, we estimate the SR% as defined by (remission/minimal manifestations status for at least 6 months using 7.5 mg or less of prednisone daily, for maintenance treatment at 2, 4, and 6 years after symptoms onset) for patients who were not taking steroid-sparing immunosuppressant (SSI) as a primary outcome and for patients taking an SSI as a secondary outcome.
Results: Forty-five patients were not taking an SSI at 2 years, 34 patients at 4 years, and 17 patients at 6 years; SR% was 44.4%, 64.7%, and 58.8%, respectively. Thirty-six patients were taking an SSI at 2 years, 22 patients at 4 years, and 15 patients at 6 years; the SR% was 50.0%, 45.4%, and 66.7%, respectively.
Conclusions: Nearly half of the generalized myasthenia gravis patients who were not taking an SSI achieved an SR.
Conflict of interest statement
Figures
References
-
- Muscle Study G. A trial of mycophenolate mofetil with prednisone as initial immunotherapy in myasthenia gravis. Neurology. 2008;71(6):394–9. - PubMed
-
- Palace J, Newsom-Davis J, Lecky B. A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Myasthenia Gravis Study Group. Neurology. 1998;50(6):1778–83. - PubMed
-
- Sanders DB, Hart IK, Mantegazza R, et al. An international, phase III, randomized trial of mycophenolate mofetil in myasthenia gravis. Neurology. 2008;71(6):400–6. - PubMed
-
- Hehir MK, Burns TM, Alpers J, Conaway MR, Sawa M, Sanders DB. Mycophenolate mofetil in AChR-antibody-positive myasthenia gravis: outcomes in 102 patients. Muscle Nerve. 2010;41(5):593–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials