Telomere length-dependent transcription and epigenetic modifications in promoters remote from telomere ends
- PMID: 30439955
- PMCID: PMC6264879
- DOI: 10.1371/journal.pgen.1007782
Telomere length-dependent transcription and epigenetic modifications in promoters remote from telomere ends
Erratum in
-
Correction: Telomere length-dependent transcription and epigenetic modifications in promoters remote from telomere ends.PLoS Genet. 2020 Oct 22;16(10):e1009152. doi: 10.1371/journal.pgen.1009152. eCollection 2020 Oct. PLoS Genet. 2020. PMID: 33091002 Free PMC article.
Abstract
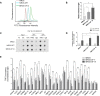
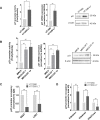
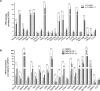
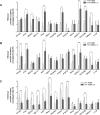
Telomere-binding proteins constituting the shelterin complex have been studied primarily for telomeric functions. However, mounting evidence shows non-telomeric binding and gene regulation by shelterin factors. This raises a key question-do telomeres impact binding of shelterin proteins at distal non-telomeric sites? Here we show that binding of the telomere-repeat-binding-factor-2 (TRF2) at promoters ~60 Mb from telomeres depends on telomere length in human cells. Promoter TRF2 occupancy was depleted in cells with elongated telomeres resulting in altered TRF2-mediated transcription of distal genes. In addition, histone modifications-activation (H3K4me1 and H3K4me3) as well as silencing marks (H3K27me3)-at distal promoters were telomere length-dependent. These demonstrate that transcription, and the epigenetic state, of telomere-distal promoters can be influenced by telomere length. Molecular links between telomeres and the extra-telomeric genome, emerging from findings here, might have important implications in telomere-related physiology, particularly ageing and cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Blackburn EH. Structure and function of telomeres. Nature. 1991;350: 569–573. 10.1038/350569a0 - DOI - PubMed
-
- Greider CW. Telomere Length Regulation. Annu Rev Biochem. Annual Reviews; 1996;65: 337–365. 10.1146/annurev.bi.65.070196.002005 - DOI - PubMed
-
- De Lange T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19: 2100–2110. 10.1101/gad.1346005 - DOI - PubMed
-
- Rhodes D, Fairall L, Simonsson T, Court R, Chapman L. Telomere architecture. EMBO Rep. 2002;3: 1139–1145. 10.1093/embo-reports/kvf246 - DOI - PMC - PubMed
-
- O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. Nature Publishing Group; 2010;11: 171–181. 10.1038/nrm2848 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

