The deletion of the ORF1 and ORF71 genes reduces virulence of the neuropathogenic EHV-1 strain Ab4 without compromising host immunity in horses
- PMID: 30440016
- PMCID: PMC6237298
- DOI: 10.1371/journal.pone.0206679
The deletion of the ORF1 and ORF71 genes reduces virulence of the neuropathogenic EHV-1 strain Ab4 without compromising host immunity in horses
Abstract
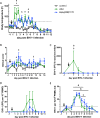
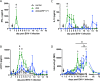
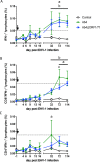
The equine herpesvirus type 1 (EHV-1) ORF1 and ORF71 genes have immune modulatory effects in vitro. Experimental infection of horses using virus mutants with multiple deletions including ORF1 and ORF71 showed promise as vaccine candidates against EHV-1. Here, the combined effects of ORF1 and ORF71 deletions from the neuropathogenic EHV-1 strain Ab4 on clinical disease and host immune response were further explored. Three groups of EHV-1 naïve horses were experimentally infected with the ORF1/71 gene deletion mutant (Ab4ΔORF1/71), the parent Ab4 strain, or remained uninfected. In comparison to Ab4, horses infected with Ab4ΔORF1/71 did not show the initial high fever peak characteristic of EHV-1 infection. Ab4ΔORF1/71 infection had reduced nasal shedding (1/5 vs. 5/5) and, simultaneously, decreased intranasal interferon (IFN)-α, interleukin (IL)-10 and soluble CD14 secretion. However, Ab4 and Ab4ΔORF1/71 infection resulted in comparable viremia, suggesting these genes do not regulate the infection of the mononuclear cells and subsequent viremia. Intranasal and serum anti-EHV-1 antibodies to Ab4ΔORF1/71 developed slightly slower than those to Ab4. However, beyond day 12 post infection (d12pi) serum antibodies in both virus-infected groups were similar and remained increased until the end of the study (d114pi). EHV-1 immunoglobulin (Ig) G isotype responses were dominated by short-lasting IgG1 and long-lasting IgG4/7 antibodies. The IgG4/7 response closely resembled the total EHV-1 specific antibody response. Ex vivo re-stimulation of PBMC with Ab4 resulted in IFN-γ and IL-10 secretion by cells from both infected groups within two weeks pi. Flow cytometric analysis showed that IFN-γ producing EHV-1-specific T-cells were mainly CD8+/IFN-γ+ and detectable from d32pi on. Peripheral blood IFN-γ+ T-cell percentages were similar in both infected groups, albeit at low frequency (~0.1%). In summary, the Ab4ΔORF1/71 gene deletion mutant is less virulent but induced antibody responses and cellular immunity similar to the parent Ab4 strain.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Viremia and nasal shedding for the diagnosis of equine herpesvirus-1 infection in domesticated horses.J Vet Intern Med. 2024 May-Jun;38(3):1765-1791. doi: 10.1111/jvim.16958. Epub 2023 Dec 9. J Vet Intern Med. 2024. PMID: 38069548 Free PMC article. Review.
-
An Equine Herpesvirus Type 1 (EHV-1) Ab4 Open Reading Frame 2 Deletion Mutant Provides Immunity and Protection from EHV-1 Infection and Disease.J Virol. 2019 Oct 29;93(22):e01011-19. doi: 10.1128/JVI.01011-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462575 Free PMC article.
-
Deletion of the ORF2 gene of the neuropathogenic equine herpesvirus type 1 strain Ab4 reduces virulence while maintaining strong immunogenicity.BMC Vet Res. 2018 Aug 22;14(1):245. doi: 10.1186/s12917-018-1563-4. BMC Vet Res. 2018. PMID: 30134896 Free PMC article.
-
Intranasal IgG4/7 antibody responses protect horses against equid herpesvirus-1 (EHV-1) infection including nasal virus shedding and cell-associated viremia.Virology. 2019 May;531:219-232. doi: 10.1016/j.virol.2019.03.014. Epub 2019 Mar 22. Virology. 2019. PMID: 30928700
-
Immune escape of equine herpesvirus 1 and other herpesviruses of veterinary importance.Vet Immunol Immunopathol. 2006 May 15;111(1-2):31-40. doi: 10.1016/j.vetimm.2006.01.006. Epub 2006 Feb 10. Vet Immunol Immunopathol. 2006. PMID: 16472872 Review.
Cited by
-
Development of a quantitative COVID-19 multiplex assay and its use for serological surveillance in a low SARS-CoV-2 incidence community.PLoS One. 2022 Jan 21;17(1):e0262868. doi: 10.1371/journal.pone.0262868. eCollection 2022. PLoS One. 2022. PMID: 35061843 Free PMC article.
-
Viremia and nasal shedding for the diagnosis of equine herpesvirus-1 infection in domesticated horses.J Vet Intern Med. 2024 May-Jun;38(3):1765-1791. doi: 10.1111/jvim.16958. Epub 2023 Dec 9. J Vet Intern Med. 2024. PMID: 38069548 Free PMC article. Review.
-
An Equine Herpesvirus Type 1 (EHV-1) Ab4 Open Reading Frame 2 Deletion Mutant Provides Immunity and Protection from EHV-1 Infection and Disease.J Virol. 2019 Oct 29;93(22):e01011-19. doi: 10.1128/JVI.01011-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462575 Free PMC article.
-
Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2.J Virol. 2021 May 10;95(11):e00083-21. doi: 10.1128/JVI.00083-21. Epub 2021 Mar 10. J Virol. 2021. PMID: 33692203 Free PMC article.
-
Increase in Virus-Specific Mucosal Antibodies in the Upper Respiratory Tract Following Intramuscular Vaccination of Previously Exposed Horses Against Equine Herpesvirus Type-1/4.Vaccines (Basel). 2025 Mar 10;13(3):290. doi: 10.3390/vaccines13030290. Vaccines (Basel). 2025. PMID: 40266191 Free PMC article.
References
-
- Lunn DP, Davis‐Poynter N, Flaminio MJ, Horohov DW, Osterrieder K, Pusterla N, et al. Equine Herpesvirus‐1 Consensus Statement. J Vet Intern Med. 2009;23: 450–61. 10.1111/j.1939-1676.2009.0304.x - DOI - PubMed
-
- Kydd JH, Smith KC, Hannant D, Livesay GJ, Mumford JA. Distribution of Equid herpesvirus‐1 (EHV‐1) in the respiratory tract of ponies: implications for vaccination strategies. Equine Vet J. 1994;1:466–9. - PubMed
-
- Slater JD, Borchers K, Thackray AM, Field HJ. The trigeminal ganglion is a location for equine herpesvirus 1 latency and reactivation in the horse. Journal of General Virology. 1994;75:2007–16. 10.1099/0022-1317-75-8-2007 - DOI - PubMed
-
- Perkins GA, Goodman LB, Tsujimura K, Van de Walle GR, Kim SG, Dubovi EJ, et al. Investigation of the prevalence of neurologic equine herpes virus type 1 (EHV-1) in a 23-year retrospective analysis (1984–2007). Vet Microbiol. 2009;139:375–8. 10.1016/j.vetmic.2009.06.033 - DOI - PubMed
-
- Smith KC, Borchers K. A study of the pathogenesis of equid herpesvirus-1 (EHV-1) abortion by DNA in-situ hybridization. J Comp Pathol. 2001;125:304–10. 10.1053/jcpa.2001.0513 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

