An Antithrombotic Strategy by Targeting Phospholipase D in Human Platelets
- PMID: 30441821
- PMCID: PMC6262437
- DOI: 10.3390/jcm7110440
An Antithrombotic Strategy by Targeting Phospholipase D in Human Platelets
Abstract
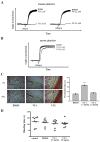
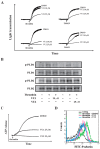
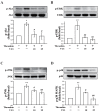
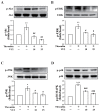
Phospholipase D (PLD) is involved in many biological processes. PLD1 plays a crucial role in regulating the platelet activity of mice; however, the role of PLD in the platelet activation of humans remains unclear. Therefore, we investigated whether PLD is involved in the platelet activation of humans. Our data revealed that inhibition of PLD1 or PLD2 using pharmacological inhibitors effectively inhibits platelet aggregation in humans. However, previous studies have showed that PLD1 or PLD2 deletion did not affect mouse platelet aggregation in vitro, whereas only PLD1 deletion inhibited thrombus formation in vivo. Intriguingly, our data also showed that the pharmacological inhibition of PLD1 or PLD2 does not affect mouse platelet aggregation in vitro, whereas the inhibition of only PLD1 delayed thrombus formation in vivo. These findings indicate that PLD may play differential roles in humans and mice. In humans, PLD inhibition attenuates platelet activation, adhesion, spreading, and clot retraction. For the first time, we demonstrated that PLD1 and PLD2 are essential for platelet activation in humans, and PLD plays different roles in platelet function in humans and mice. Our findings also indicate that targeting PLD may provide a safe and alternative therapeutic approach for preventing thromboembolic disorders.
Keywords: clot retraction; phospholipase D; platelet activation; thrombus formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Phospholipase D1 and D2 Synergistically Regulate Thrombus Formation.Int J Mol Sci. 2020 Sep 22;21(18):6954. doi: 10.3390/ijms21186954. Int J Mol Sci. 2020. PMID: 32971863 Free PMC article.
-
Redundant functions of phospholipases D1 and D2 in platelet α-granule release.J Thromb Haemost. 2012 Nov;10(11):2361-72. doi: 10.1111/j.1538-7836.2012.04924.x. J Thromb Haemost. 2012. PMID: 22974101
-
Effects of phospholipase D during cultured osteoblast mineralization and bone formation.J Cell Biochem. 2019 Apr;120(4):5923-5935. doi: 10.1002/jcb.27881. Epub 2018 Oct 15. J Cell Biochem. 2019. PMID: 30320913
-
Expression of phospholipase D isoforms in mammalian cells.Biochim Biophys Acta. 1999 Jul 30;1439(2):199-213. doi: 10.1016/s1388-1981(99)00095-5. Biochim Biophys Acta. 1999. PMID: 10425396 Review.
-
The phospholipase D superfamily as therapeutic targets.Trends Pharmacol Sci. 2015 Mar;36(3):137-44. doi: 10.1016/j.tips.2015.01.001. Epub 2015 Feb 3. Trends Pharmacol Sci. 2015. PMID: 25661257 Free PMC article. Review.
Cited by
-
Phospholipase D1 and D2 Synergistically Regulate Thrombus Formation.Int J Mol Sci. 2020 Sep 22;21(18):6954. doi: 10.3390/ijms21186954. Int J Mol Sci. 2020. PMID: 32971863 Free PMC article.
-
Integrin αIIbβ3 outside-in signaling activates human platelets through serine 24 phosphorylation of Disabled-2.Cell Biosci. 2021 Feb 8;11(1):32. doi: 10.1186/s13578-021-00532-5. Cell Biosci. 2021. PMID: 33557943 Free PMC article.
References
-
- Collaboration A.T.A., Baigent C., Blackwell L., Collins R., Emberson J., Godwin J., Peto R., Buring J., Hennekens C., Kearney P., et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. - PMC - PubMed
-
- Elvers M., Stegner D., Hagedorn I., Kleinschnitz C., Braun A., Kuijpers M.E., Boesl M., Chen Q., Heemskerk J.W., Stoll G., et al. Impaired alpha(IIb)beta(3) integrin activation and shear-dependent thrombus formation in mice lacking phospholipase D1. Sci. Signal. 2010;3:ra1. doi: 10.1126/scisignal.2000551. - DOI - PMC - PubMed
Grants and funding
- MOST104-2320-B-038-045-MY2/Ministry of Science and Technology, Taiwan
- MOST105-2320-B-341-001/Ministry of Science and Technology, Taiwan
- MOST105-2311-B-038-005-MY3/Ministry of Science and Technology, Taiwan
- MOST106-2320-B-715-006-MY3/Ministry of Science and Technology, Taiwan
- TMU105-AE-B01/Taipei Medical University
LinkOut - more resources
Full Text Sources

