Emerging Role of Purine Metabolizing Enzymes in Brain Function and Tumors
- PMID: 30441833
- PMCID: PMC6274932
- DOI: 10.3390/ijms19113598
Emerging Role of Purine Metabolizing Enzymes in Brain Function and Tumors
Abstract
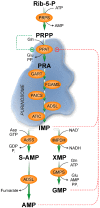
The growing evidence of the involvement of purine compounds in signaling, of nucleotide imbalance in tumorigenesis, the discovery of purinosome and its regulation, cast new light on purine metabolism, indicating that well known biochemical pathways may still surprise. Adenosine deaminase is important not only to preserve functionality of immune system but also to ensure a correct development and function of central nervous system, probably because its activity regulates the extracellular concentration of adenosine and therefore its function in brain. A lot of work has been done on extracellular 5'-nucleotidase and its involvement in the purinergic signaling, but also intracellular nucleotidases, which regulate the purine nucleotide homeostasis, play unexpected roles, not only in tumorigenesis but also in brain function. Hypoxanthine guanine phosphoribosyl transferase (HPRT) appears to have a role in the purinosome formation and, therefore, in the regulation of purine synthesis rate during cell cycle with implications in brain development and tumors. The final product of purine catabolism, uric acid, also plays a recently highlighted novel role. In this review, we discuss the molecular mechanisms underlying the pathological manifestations of purine dysmetabolisms, focusing on the newly described/hypothesized roles of cytosolic 5'-nucleotidase II, adenosine kinase, adenosine deaminase, HPRT, and xanthine oxidase.
Keywords: adenosine deaminase; adenosine kinase; cytosolic 5′-nucleotidase II; hypoxanthine guanine phosphoribosyl transferase; uric acid; xanthine oxidase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Zhou G., Smith J.L., Zalkin H. Binding of purine nucleotides to two regulatory sites results in synergistic feedback inhibition of glutamine 5-phosphoribosylpyrophosphate amidotransferase. J. Biol. Chem. 1994;269:6784–6789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

