Gadolinium Chloride Rescues Niemann⁻Pick Type C Liver Damage
- PMID: 30441844
- PMCID: PMC6274821
- DOI: 10.3390/ijms19113599
Gadolinium Chloride Rescues Niemann⁻Pick Type C Liver Damage
Abstract
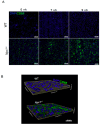
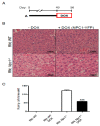
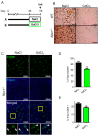
Niemann⁻Pick type C (NPC) disease is a rare neurovisceral cholesterol storage disorder that arises from loss of function mutations in the NPC1 or NPC2 genes. Soon after birth, some patients present with an aggressive hepatosplenomegaly and cholestatic signs. Histopathologically, the liver presents with large numbers of foam cells; however, their role in disease pathogenesis has not been explored in depth. Here, we studied the consequences of gadolinium chloride (GdCl₃) treatment, a well-known Kupffer/foam cell inhibitor, at late stages of NPC liver disease and compared it with NPC1 genetic rescue in hepatocytes in vivo. GdCl₃ treatment successfully blocked the endocytic capacity of hepatic Kupffer/foam measured by India ink endocytosis, decreased the levels CD68-A marker of Kupffer cells in the liver-and normalized the transaminase levels in serum of NPC mice to a similar extent to those obtained by genetic Npc1 rescue of liver cells. Gadolinium salts are widely used as magnetic resonance imaging (MRI) contrasts. This study opens the possibility of targeting foam cells with gadolinium or by other means for improving NPC liver disease. Synopsis: Gadolinium chloride can effectively rescue some parameters of liver dysfunction in NPC mice and its potential use in patients should be carefully evaluated.
Keywords: Niemann–Pick type C disease; cholesterol; gadolinium chloride; liver disease; therapeutic option.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Complement Component C3 Participates in Early Stages of Niemann-Pick C Mouse Liver Damage.Int J Mol Sci. 2020 Mar 20;21(6):2127. doi: 10.3390/ijms21062127. Int J Mol Sci. 2020. PMID: 32244854 Free PMC article.
-
Lysosomal vitamin E accumulation in Niemann-Pick type C disease.Biochim Biophys Acta. 2012 Feb;1822(2):150-60. doi: 10.1016/j.bbadis.2011.11.009. Epub 2011 Nov 15. Biochim Biophys Acta. 2012. PMID: 22120593
-
Superoxide produced by Kupffer cells is an essential effector in concanavalin A-induced hepatitis in mice.Hepatology. 2008 Dec;48(6):1979-88. doi: 10.1002/hep.22561. Hepatology. 2008. PMID: 18942689
-
Complex lipid trafficking in Niemann-Pick disease type C.J Inherit Metab Dis. 2015 Jan;38(1):187-99. doi: 10.1007/s10545-014-9794-4. Epub 2014 Nov 26. J Inherit Metab Dis. 2015. PMID: 25425283 Review.
-
[Adult onset Niemann-Pick type C disease and psychosis: literature review].Encephale. 2013 Oct;39(5):315-9. doi: 10.1016/j.encep.2013.04.013. Epub 2013 Aug 5. Encephale. 2013. PMID: 23928063 Review. French.
Cited by
-
Complement Component C3 Participates in Early Stages of Niemann-Pick C Mouse Liver Damage.Int J Mol Sci. 2020 Mar 20;21(6):2127. doi: 10.3390/ijms21062127. Int J Mol Sci. 2020. PMID: 32244854 Free PMC article.
-
Deficiency of myeloid NPC1 exacerbates liver injury and fibrosis by impairing macrophage efferocytosis.J Adv Res. 2025 Jun;72:213-227. doi: 10.1016/j.jare.2024.11.020. Epub 2024 Nov 14. J Adv Res. 2025. PMID: 39547438 Free PMC article.
-
Understanding and Treating Niemann-Pick Type C Disease: Models Matter.Int J Mol Sci. 2020 Nov 26;21(23):8979. doi: 10.3390/ijms21238979. Int J Mol Sci. 2020. PMID: 33256121 Free PMC article. Review.
-
Advances in research on potential therapeutic approaches for Niemann-Pick C1 disease.Front Pharmacol. 2024 Aug 28;15:1465872. doi: 10.3389/fphar.2024.1465872. eCollection 2024. Front Pharmacol. 2024. PMID: 39263569 Free PMC article. Review.
-
Aging results in accumulation of M1 and M2 hepatic macrophages and a differential response to gadolinium chloride.Histochem Cell Biol. 2020 Jan;153(1):37-48. doi: 10.1007/s00418-019-01827-y. Epub 2019 Nov 6. Histochem Cell Biol. 2020. PMID: 31691025
References
-
- Klein A.D., Alvarez A., Zanlungo S. The unique case of the Niemann–Pick type C cholesterol storage disorder. Pediatr. Endocrinol. Rev. 2014;12:166–175. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

