Small Molecules Enhance Scaffold-Based Bone Grafts via Purinergic Receptor Signaling in Stem Cells
- PMID: 30441872
- PMCID: PMC6274752
- DOI: 10.3390/ijms19113601
Small Molecules Enhance Scaffold-Based Bone Grafts via Purinergic Receptor Signaling in Stem Cells
Abstract
The need for bone grafts is high, due to age-related diseases, such as tumor resections, but also accidents, risky sports, and military conflicts. The gold standard for bone grafting is the use of autografts from the iliac crest, but the limited amount of accessible material demands new sources of bone replacement. The use of mesenchymal stem cells or their descendant cells, namely osteoblast, the bone-building cells and endothelial cells for angiogenesis, combined with artificial scaffolds, is a new approach. Mesenchymal stem cells (MSCs) can be obtained from the patient themselves, or from donors, as they barely cause an immune response in the recipient. However, MSCs never fully differentiate in vitro which might lead to unwanted effects in vivo. Interestingly, purinergic receptors can positively influence the differentiation of both osteoblasts and endothelial cells, using specific artificial ligands. An overview is given on purinergic receptor signaling in the most-needed cell types involved in bone metabolism-namely osteoblasts, osteoclasts, and endothelial cells. Furthermore, different types of scaffolds and their production methods will be elucidated. Finally, recent patents on scaffold materials, as wells as purinergic receptor-influencing molecules which might impact bone grafting, are discussed.
Keywords: angiogenesis; bone; drug release; mesenchymal stem cells; osteoblast; osteoclast; patent; purinergic receptors; scaffold.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

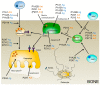


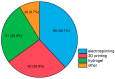
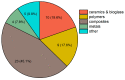
References
-
- Burk T., Del Valle J., Finn R.A., Phillips C. Maximum Quantity of Bone Available for Harvest From the Anterior Iliac Crest, Posterior Iliac Crest, and Proximal Tibia Using a Standardized Surgical Approach: A Cadaveric Study. J. Oral Maxillofac. Surg. 2016;74:2532–2548. doi: 10.1016/j.joms.2016.06.191. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 13FH012PB2/Bundesministerium für Bildung und Forschung
- 1720X06/Bundesministerium für Bildung und Forschung
- 03FH019IX5/Bundesministerium für Bildung und Forschung
- z1112fh012/Ministerium für Innovation, Wissenschaft und Forschung des Landes Nordrhein-Westfalen
- 314-vigoni-dr/Deutscher Akademischer Austauschdienst
LinkOut - more resources
Full Text Sources

