Identification of novel LEPR mutations in Pakistani families with morbid childhood obesity
- PMID: 30442103
- PMCID: PMC6238292
- DOI: 10.1186/s12881-018-0710-x
Identification of novel LEPR mutations in Pakistani families with morbid childhood obesity
Abstract
Background: Mutations in the genes encoding leptin (LEP), the leptin receptor (LEPR), and the melanocortin 4 receptor (MC4R) are known to cause severe early-onset childhood obesity. The aim of the current study was to examine the prevalence of damaging LEP, LEPR, and MC4R mutations in Pakistani families having a recessive heritance of early-onset obesity.
Methods: Using targeted resequencing, the presence of rare mutations in LEP, LEPR, and MC4R, was investigated in individuals from 25 families suspected of having autosomal recessive early-onset obesity. Segregation patterns of variants were assessed based on chip-based genotyping.
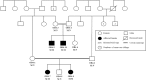
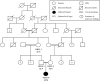
Results: Homozygous LEPR variants were identified in two probands. One carried a deletion (c.3260AG) resulting in the frameshift mutation p.Ser1090Trpfs*6, and the second carried a substitution (c.2675C > G) resulting in the missense mutation p.Pro892Arg. Both mutations were located within regions of homozygosity shared only among affected individuals. Both probands displayed early-onset obesity, hyperphagia and diabetes. No mutations were found in LEP and MC4R.
Conclusions: The current study highlights the implication of LEPR mutations in cases of severe early-onset obesity in consanguineous Pakistani families. Through targeted resequencing, we identified novel damaging mutations, and our approach may therefore be utilized in clinical testing or diagnosis of known forms of monogenic obesity with the aim of optimizing obesity treatment.
Keywords: Early-onset obesity; Hyperphagia; Leptin; Leptin receptor; Melanocortin 4 receptor; Monogenic obesity; Pakistani families; Targeted resequencing.
Conflict of interest statement
Ethics approval and consent to participate
The current study protocol was approved by the local Ethical Committee with the name of ‘Ethics Review Board (ERB) of Pakistan Institute of Medical Sciences (PIMS) at Shaheed Zulfiqar Ali Bhutto Medica University (SZABMU), Islamabad, Pakistan and the committee’s reference number is No. F. 1–1/2015/ERB/SZABMU/−. The study was conducted in accordance with the guidelines of the Helsinki Declaration. Informed consent forms were obtained from individuals > 18 years, while informed consent for individuals < 18 years of age were given by parents or guardians.
Consent for publication
Written informed consent was obtained from the patients or their parents for the publication of their clinical and genetic data.
Competing interests
Wasim Ahmad is a member of the editorial board (Associate Editor) of BMC Medical Genetics. The remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous