Feasibility of the string test for tuberculosis diagnosis in children between 4 and 14 years old
- PMID: 30442105
- PMCID: PMC6238308
- DOI: 10.1186/s12879-018-3483-0
Feasibility of the string test for tuberculosis diagnosis in children between 4 and 14 years old
Abstract
Background: The enteric string test can be used to obtain a specimen for microbiological confirmation of tuberculosis in children, but it is not widely used for this. The aim of this analysis to evaluate this approach in children with tuberculosis symptoms.
Methods: We conducted a cross-sectional study to assess children's ability to complete the test (feasibility), and self-reported pain (tolerability). We examined caregivers' and children's willingness to repeat the procedure (acceptability) and described the diagnostic yield of cultures for diagnostic tools. We stratified estimates by age and compared metrics to those derived for gastric aspirate (GA).
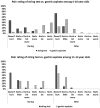
Results: Among 148 children who attempted the string test, 34% successfully swallowed the capsule. Feasibility was higher among children aged 11-14 than in children 4-10 years (83% vs 22% respectively, p < 0.0001). The string test was better tolerated than GA in both age groups; however, guardians and older children reported higher rates of willingness to repeat GA than the string test (86% vs. 58% in children; 100% vs. 83% in guardians). In 9 children with a positive sputum culture, 6 had a positive string culture. The one children with a positive gastric aspirate culture also had a positive string culture.
Conclusion: Although the string test was generally tolerable and accepted by children and caregivers; feasibility in young children was low. Reducing the capsule size may improve test success rates in younger children.
Keywords: Feasibility; Gastric aspirate; Pediatric; Peru; Tolerability.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Peru’s National Institute of Health and the Office of Human Research Administration at the Harvard Medical School. Guardians provided written informed consent, and children eight years of age and older provided written informed assent.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Feasibility and utility of a combined nasogastric-tube-and-string-test device for bacteriologic confirmation of pulmonary tuberculosis in young children.Diagn Microbiol Infect Dis. 2024 Jul;109(3):116302. doi: 10.1016/j.diagmicrobio.2024.116302. Epub 2024 Apr 18. Diagn Microbiol Infect Dis. 2024. PMID: 38657352 Free PMC article.
-
Detection Yield and Tolerability of String Test for Diagnosis of Childhood Intrathoracic Tuberculosis.Pediatr Infect Dis J. 2016 Feb;35(2):146-51. doi: 10.1097/INF.0000000000000956. Pediatr Infect Dis J. 2016. PMID: 26517328
-
Xpert MTB/RIF assay can be used on archived gastric aspirate and induced sputum samples for sensitive diagnosis of paediatric tuberculosis.BMC Microbiol. 2015 Sep 29;15:191. doi: 10.1186/s12866-015-0528-z. BMC Microbiol. 2015. PMID: 26420261 Free PMC article.
-
Laboratory Diagnosis of Mycobacterium tuberculosis Infection and Disease in Children.J Clin Microbiol. 2016 Jun;54(6):1434-1441. doi: 10.1128/JCM.03043-15. Epub 2016 Mar 16. J Clin Microbiol. 2016. PMID: 26984977 Free PMC article. Review.
-
The diagnosis of tuberculosis.Pediatr Infect Dis J. 2012 Mar;31(3):302-5. doi: 10.1097/INF.0b013e318249f26d. Pediatr Infect Dis J. 2012. PMID: 22330167 Review.
Cited by
-
Incident Tuberculosis Diagnoses in Children at High Risk for Disease.Open Forum Infect Dis. 2021 Feb 18;8(3):ofab075. doi: 10.1093/ofid/ofab075. eCollection 2021 Mar. Open Forum Infect Dis. 2021. PMID: 33738322 Free PMC article.
-
Is exclusive breastfeeding for six-months protective against pediatric tuberculosis?Glob Health Action. 2021 Jan 1;14(1):1861922. doi: 10.1080/16549716.2020.1861922. Glob Health Action. 2021. PMID: 33393436 Free PMC article.
-
Diagnostic accuracy of molecular detection of Mycobacterium tuberculosis in pediatric stool samples: A systematic review and meta-analysis.Tuberculosis (Edinb). 2019 Dec;119:101878. doi: 10.1016/j.tube.2019.101878. Epub 2019 Oct 23. Tuberculosis (Edinb). 2019. PMID: 31670064 Free PMC article.
-
Sampling strategies for digestive system flora studies: current research and perspectives.PeerJ. 2025 Aug 13;13:e19810. doi: 10.7717/peerj.19810. eCollection 2025. PeerJ. 2025. PMID: 40821994 Free PMC article. Review.
-
Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD013359. doi: 10.1002/14651858.CD013359.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2022 Sep 6;9:CD013359. doi: 10.1002/14651858.CD013359.pub3. PMID: 32853411 Free PMC article. Updated.
References
-
- WHO . Global Tuberculosis Report 2018. Switzerland: World Health Organization; 2018.
-
- WHO . Guidance for national tubeculosis programmes on the managment of tuberculosis in children. Switzerland: World Health Organization; 2014. - PubMed
-
- Mukherjee A, Singh S, Lodha R, Singh V, Hesseling AC, Grewal HM, et al. Ambulatory gastric lavages provide better yields of Mycobacterium tuberculosis than induced sputum in children with intrathoracic tuberculosis. Pediatr Infect Dis J. 2013;32(12):1313–1317. doi: 10.1097/INF.0b013e31829f5c58. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical