Modeling hallmark pathology using motor neurons derived from the family and sporadic amyotrophic lateral sclerosis patient-specific iPS cells
- PMID: 30442180
- PMCID: PMC6238404
- DOI: 10.1186/s13287-018-1048-1
Modeling hallmark pathology using motor neurons derived from the family and sporadic amyotrophic lateral sclerosis patient-specific iPS cells
Erratum in
-
Correction to: Modeling hallmark pathology using motor neurons derived from the family and sporadic amyotrophic lateral sclerosis patient-specific iPS cells.Stem Cell Res Ther. 2019 Mar 15;10(1):97. doi: 10.1186/s13287-019-1211-3. Stem Cell Res Ther. 2019. PMID: 30876443 Free PMC article.
Abstract
Background: Amyotrophic lateral sclerosis (ALS) represents a devastating, progressive, heterogeneous, and the most common motor neuron (MN) disease. To date, no cure has been available for the condition. Studies with transgenic mice have yielded significant results that help us understand the underlying mechanisms of ALS. Nonetheless, none of more than 30 large clinical trials over the past 20 years proved successful, which led some researchers to challenge the validity of the preclinical models.
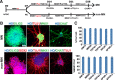
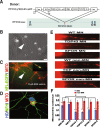
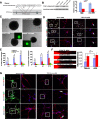
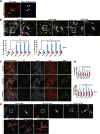
Methods: Human-induced pluripotent cells (iPSCs) were established by introducing Sendai virus into fibroblast cells. We established TDP-43 HES by inserting CAG-TDP43 (G298S) cassette or the CAG-EGFP cassette into PPP1R12C-locus of human embryonic stem cells (ESC, H9) by TALEN-mediated homologous recombination. iPSCs or HESC were differentiated to motor neurons and non-motor neuron as control. Relevant biomarkers were detected in different differentiated stages. TDP-43 aggregates, neurofilament, and mitochondria analyses were performed.
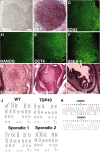
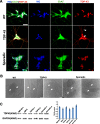
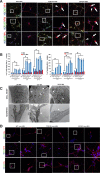
Results: In this study, using iPSCs-derived human MN from an ALS patient with a TDP43 G298S mutation and two sporadic ALS patients, we showed that both sporadic and familial ALS were characterized by TDP-43 aggregates in the surviving MN. Significantly higher neurofilament (NF) inclusion was also found in ALS MN compared with wild-type (WT) GM15 controls (P < 0.05). The neurite mitochondria density was significantly lower in ALS MN than that in the control MNs. Transgenesis of TDP-43 G298S into AAVS locus in human embryonic stem cells reproduced phenotype of patient-derived G289S MN. By challenging MNs with a proteasome inhibitor, we found that MNs were more vulnerable to MG132, with some accompanying phenotype changes, such as TDP43 translocation, NF inclusion, mitochondria distribution impairment, and activation of caspase3.
Conclusions: Our results suggested that changes in TDP43 protein, NF inclusion, and distribution impairment of mitochondria are common early pathology both in familial and sporadic ALS. These findings will help us gain insight into the pathogenesis of the condition and screen relevant drugs for the disease.
Keywords: Amyotrophic lateral sclerosis; Induced pluripotent cells; Mitochondria; Neurofilament; TDP-43.
Conflict of interest statement
Ethics approval
Skin biopsy were harvested from male patients 61–72 years old whose relatives gave informed consent for the study
All animal experiments were conducted in accordance with the Guide for the Care and Use of Animals for Research Purposes. The study protocols were approved by Institutional Review Board Approval of Experimental Animals of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Permit Number: TJ-IBR20130212).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
A cellular model for sporadic ALS using patient-derived induced pluripotent stem cells.Mol Cell Neurosci. 2013 Sep;56:355-64. doi: 10.1016/j.mcn.2013.07.007. Epub 2013 Jul 25. Mol Cell Neurosci. 2013. PMID: 23891805 Free PMC article.
-
Nicotinamide Adenine Dinucleotide Precursor Supplementation Modulates Neurite Complexity and Survival in Motor Neurons from Amyotrophic Lateral Sclerosis Models.Antioxid Redox Signal. 2024 Sep;41(7-9):573-589. doi: 10.1089/ars.2023.0360. Epub 2024 Jul 8. Antioxid Redox Signal. 2024. PMID: 38504592
-
[ALS disease modeling and drug screening using patient-specific iPS cells].Rinsho Shinkeigaku. 2013;53(11):1020-2. doi: 10.5692/clinicalneurol.53.1020. Rinsho Shinkeigaku. 2013. PMID: 24291866 Review. Japanese.
-
Progressive Motor Neuron Pathology and the Role of Astrocytes in a Human Stem Cell Model of VCP-Related ALS.Cell Rep. 2017 May 30;19(9):1739-1749. doi: 10.1016/j.celrep.2017.05.024. Cell Rep. 2017. PMID: 28564594 Free PMC article.
-
Reverse engineering human neurodegenerative disease using pluripotent stem cell technology.Brain Res. 2016 May 1;1638(Pt A):30-41. doi: 10.1016/j.brainres.2015.09.023. Epub 2015 Sep 28. Brain Res. 2016. PMID: 26423934 Free PMC article. Review.
Cited by
-
HDAC6 inhibition restores TDP-43 pathology and axonal transport defects in human motor neurons with TARDBP mutations.EMBO J. 2021 Apr 1;40(7):e106177. doi: 10.15252/embj.2020106177. Epub 2021 Mar 10. EMBO J. 2021. PMID: 33694180 Free PMC article.
-
Induced Pluripotent Stem Cells and Their Applications in Amyotrophic Lateral Sclerosis.Cells. 2023 Mar 22;12(6):971. doi: 10.3390/cells12060971. Cells. 2023. PMID: 36980310 Free PMC article. Review.
-
Motor neuron-derived induced pluripotent stem cells as a drug screening platform for amyotrophic lateral sclerosis.Front Cell Dev Biol. 2022 Aug 24;10:962881. doi: 10.3389/fcell.2022.962881. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36105357 Free PMC article. Review.
-
Intra-condensate demixing of TDP-43 inside stress granules generates pathological aggregates.bioRxiv [Preprint]. 2024 May 24:2024.01.23.576837. doi: 10.1101/2024.01.23.576837. bioRxiv. 2024. Update in: Cell. 2025 Jul 24;188(15):4123-4140.e18. doi: 10.1016/j.cell.2025.04.039. PMID: 38328053 Free PMC article. Updated. Preprint.
-
KIF1A, R1457Q, and P1688L Mutations Induce Protein Abnormal Aggregation and Autophagy Impairment in iPSC-Derived Motor Neurons.Biomedicines. 2024 Jul 30;12(8):1693. doi: 10.3390/biomedicines12081693. Biomedicines. 2024. PMID: 39200158 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

