Oral polio vaccine response in the MAL-ED birth cohort study: Considerations for polio eradication strategies
- PMID: 30442479
- PMCID: PMC6325791
- DOI: 10.1016/j.vaccine.2018.05.080
Oral polio vaccine response in the MAL-ED birth cohort study: Considerations for polio eradication strategies
Abstract
Background: Immunization programs have leveraged decades of research to maximize oral polio vaccine (OPV) response. Moving toward global poliovirus eradication, the WHO recommended phased OPV-to-IPV replacement on schedules in 2012. Using the MAL-ED prospective birth cohort data, we evaluated the influence of early life exposures impacting OPV immunization by measuring OPV response for serotypes 1 and 3.
Methods: Polio neutralizing antibody assays were conducted at 7 and 15 months of age for serotypes 1 and 3. Analyses were conducted on children receiving ≥3 OPV doses (n = 1449). History of vaccination, feeding patterns, physical growth, home environment, diarrhea, enteropathogen detection, and gut inflammation were examined as risk factors for non-response [Log2(titer) < 3] and Log2(titer) by serotype using multivariate regression.
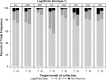
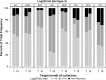
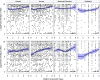
Findings: Serotype 1 seroconversion was significantly higher than serotype 3 (96.6% vs. 89.6%, 15 months). Model results indicate serotypes 1 and 3 failure was minimized following four and six OPV doses, respectively; however, enteropathogen detection and poor socioeconomic conditions attenuated response in both serotypes. At three months of age, bacterial detection in stool reduced serotype 1 and 3 Log2 titers by 0.34 (95% CI 0.14-0.54) and 0.53 (95% CI 0.29-0.77), respectively, and increased odds of serotype 3 failure by 3.0 (95% CI 1.6-5.8). Our socioeconomic index, consisting of Water, Assets, Maternal education, and Income (WAMI), was associated with a 0.79 (95% CI 0.15-1.43) and 1.23 (95% CI 0.34-2.12) higher serotype 1 and 3 Log2 titer, respectively, and a 0.04 (95% CI 0.002-0.40) lower odds of serotype 3 failure. Introduction of solids, transferrin receptor, and underweight were differentially associated with serotype response. Other factors, including diarrheal frequency and breastfeeding practices, were not associated with OPV response.
Interpretation: Under real-world conditions, improved vaccination coverage and socio-environmental conditions, and reducing early life bacterial exposures are key to improving OPV response and should inform polio eradication strategies.
Keywords: Enteropathogen infection; Home environment; Oral polio vaccination; Poliomyelitis.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures




References
-
- Global Polio Eradication Initiative. Polio cases worldwide: global polio eradication initiative; 2018. Available from: <http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Polioca....
-
- PolioEradication.Org. Circulating vaccine derived poliovirus; 2017. Available from: <http://polioeradication.org/polio-today/polio-now/this-week/circulating....
-
- Sutter R.W., Platt L., Mach O., Jafari H., Aylward R.B. The new polio eradication end game: rationale and supporting evidence. J Infect Dis. 2014;210(Suppl 1):S434–S438. - PubMed
-
- Domok I. Experiences associated with the use of live poliovirus vaccine in Hungary, 1959–1982. Rev Infect Dis. 1984;6(Suppl 2):S413–S418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

