Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503)
- PMID: 30442588
- PMCID: PMC6396275
- DOI: 10.1016/S2213-2600(18)30411-9
Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503)
Abstract
Background: Increased detection of small-sized, peripheral, non-small-cell lung cancer has renewed interest in sublobar resection instead of lobectomy, the traditional standard of care for early-stage lung cancer. We aimed to assess morbidity and mortality associated with lobar and sublobar resection for early-stage lung cancer.
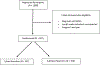
Methods: CALGB/Alliance 140503 is a multicentre, international, non-inferiority, phase 3 trial in patients with peripheral non-small-cell lung cancer clinically staged as T1aN0. Patients were recruited from 69 academic and community-based institutions in Australia, Canada, and the USA. Patients were randomly assigned intraoperatively to either lobar or sublobar resection. The random assignment was based on permuted block randomisation without concealment and was stratified according to radiographic tumour size, histology, and smoking status. The primary endpoint of the trial is disease-free survival; here, we report a post-hoc, exploratory, comparative analysis of perioperative mortality and morbidity associated with lobar and sublobar resection. Perioperative mortality was defined as death from any cause within 30 days and 90 days of surgical intervention and was calculated for all randomised patients. Morbidity was graded using Common Terminology Criteria for Adverse Events version 4.0. All analyses were done on an intention-to-treat basis for randomised patients with data available. This trial is registered with ClinicalTrials.gov, number NCT00499330.
Findings: Between June 15, 2007, and March 13, 2017, 697 patients were randomly allocated to either lobar resection (n=357) or sublobar resection (n=340; 59% wedge resection). Six (0·9%) patients died by 30 days, four (1·1%) after lobar resection and two (0·6%) after sublobar resection; by 90 days, ten (1·4%) patients had died, six (1·7%) after lobar resection and four (1·2%) after sublobar resection (difference at 30 days, 0·5%, 95% CI -1·1 to 2·3; difference at 90 days, 0·5%, 95% CI -1·5 to 2·6). An adverse event of any grade occurred in 193 (54%) of 355 patients after lobar resection and 172 (51%) of 337 patients after sublobar resection. Adverse events of grade 3 or worse occurred in 54 (15%) patients assigned lobar resection and in 48 (14%) patients assigned sublobar resection. No differences between surgical approaches were noted in cardiac or pulmonary complications. Grade 3 haemorrhage (requiring transfusion) occurred in six (2%) patients assigned lobar resection and eight (2%) patients assigned sublobar resection. Prolonged air leak occurred in nine (3%) patients after lobar resection and two (1%) patients after sublobar resection.
Interpretation: Our post-hoc analysis showed that perioperative mortality and morbidity did not seem to differ between lobar and sublobar resection in physically and functionally fit patients with clinical T1aN0 non-small-cell lung cancer. These data may affect the daily choices made by patients and their doctors in establishing the best treatment approach for stage I lung cancer.
Funding: National Cancer Institute.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT:
NA declared grants from The Cancer Therapy Evaluation Program of the National Cancer Institute (CTEP), for the Alliance for Clinical Trials in Oncology, for the conduct of this study and a grant form Astra-Zeneca for conduct of a clinical trial outside the scope of the current work.
D.R.J. is supported, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748 SR declared serving on advisory boards of Amgen, AstraZeneca, Abbvie, BMS, Lilly, Celgene, Genetech, Novartis, Takeda, Roche and Merck.
LK declared a grant from Carefusion for an unrelated study.
The authors declared no relevant conflicts of interest.
Comment in
-
A new low represents a new high in surgical safety.Lancet Respir Med. 2018 Dec;6(12):888-889. doi: 10.1016/S2213-2600(18)30465-X. Epub 2018 Nov 12. Lancet Respir Med. 2018. PMID: 30442590 No abstract available.
-
Encouraging early outcomes in cancer and leukemia group B (CALGB)/Alliance 140503: patient selection, not extent of resection, is the key to perioperative success.Ann Transl Med. 2019 Mar;7(Suppl 1):S50. doi: 10.21037/atm.2019.03.12. Ann Transl Med. 2019. PMID: 31032329 Free PMC article. No abstract available.
-
Early-stage non-small cell lung cancer: the required type of resection (lobar vs. sublobar) remains unanswered.Ann Transl Med. 2019 May;7(9):191. doi: 10.21037/atm.2019.03.56. Ann Transl Med. 2019. PMID: 31205909 Free PMC article. No abstract available.
-
Lobar or sublobar resections are safe procedures for management of early lung cancer.Ann Transl Med. 2019 Jul;7(Suppl 3):S107. doi: 10.21037/atm.2019.05.13. Ann Transl Med. 2019. PMID: 31576314 Free PMC article. No abstract available.
References
-
- Ginsberg RJ1, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995. September;60(3):615–22. - PubMed
-
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality inthe United States. N Engl J Med 2002;346:1128–37. - PubMed
-
- Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB. The influence ofhospital volume on survival after resection for lung cancer. N Engl J Med 2001;345:181–8. - PubMed
-
- Birkmeyer JD, Sun Y, Goldfaden A, Birkmeyer NJ, Stukel TA. Volume and process of care in high-risk cancer surgery. Cancer 2006;106:2476–81. Are surgical outcomes for lung cancer resections improved at teaching hospitals? - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical