Impact of High-Intensity Ultrasound on Strength of Surgical Mesh When Treating Biofilm Infections
- PMID: 30442604
- PMCID: PMC6378954
- DOI: 10.1109/TUFFC.2018.2881358
Impact of High-Intensity Ultrasound on Strength of Surgical Mesh When Treating Biofilm Infections
Abstract
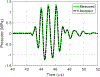
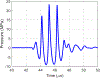
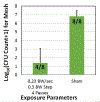
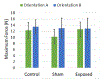
The use of cavitation-based ultrasound histotripsy to treat infections on surgical mesh has shown great potential. However, any impact of the therapy on the mesh must be assessed before the therapy can be applied in the clinic. The goal of this study was to determine if the cavitation-based therapy would reduce the strength of the mesh thus compromising the functionality of the mesh. First, Staphylococcus aureus biofilms were grown on the surgical mesh samples and exposed to high-intensity ultrasound pulses. For each exposure, the effectiveness of the therapy was confirmed by counting the number of colony forming units (CFUs) on the mesh. Most of the exposed meshes had no CFUs with an average reduction of 5.4-log10 relative to the sham exposures. To quantify the impact of the exposure on mesh strength, the force required to tear the mesh and the maximum mesh expansion before damage were quantified for control, sham, and exposed mesh samples. There was no statistical difference between the exposed and sham/control mesh samples in terms of ultimate tensile strength and corresponding mesh expansion. The only statistical difference was with respect to mesh orientation relative to the applied load. The tensile strength increased by 1.36 N while the expansion was reduced by 1.33 mm between different mesh orientations.
Figures




Similar articles
-
Histotripsy Treatment of S. Aureus Biofilms on Surgical Mesh Samples Under Varying Scan Parameters.IEEE Trans Ultrason Ferroelectr Freq Control. 2018 Jun;65(6):1017-1024. doi: 10.1109/TUFFC.2018.2819363. IEEE Trans Ultrason Ferroelectr Freq Control. 2018. PMID: 29856719 Free PMC article.
-
Histotripsy Treatment of S. Aureus Biofilms on Surgical Mesh Samples Under Varying Pulse Durations.IEEE Trans Ultrason Ferroelectr Freq Control. 2017 Oct;64(10):1420-1428. doi: 10.1109/TUFFC.2017.2718841. Epub 2017 Jun 22. IEEE Trans Ultrason Ferroelectr Freq Control. 2017. PMID: 28650808 Free PMC article.
-
Scan Parameter Optimization for Histotripsy Treatment of S. Aureus Biofilms on Surgical Mesh.IEEE Trans Ultrason Ferroelectr Freq Control. 2020 Feb;67(2):341-349. doi: 10.1109/TUFFC.2019.2948305. Epub 2019 Oct 18. IEEE Trans Ultrason Ferroelectr Freq Control. 2020. PMID: 31634828 Free PMC article.
-
Mesh-related infections after hernia repair surgery.Clin Microbiol Infect. 2005 Jan;11(1):3-8. doi: 10.1111/j.1469-0691.2004.01014.x. Clin Microbiol Infect. 2005. PMID: 15649297 Review.
-
Mesh Infection and Hernia Repair: A Review.Surg Infect (Larchmt). 2016 Apr;17(2):124-37. doi: 10.1089/sur.2015.078. Epub 2015 Dec 10. Surg Infect (Larchmt). 2016. PMID: 26654576 Review.
Cited by
-
Histotripsy: the first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound.Int J Hyperthermia. 2021;38(1):561-575. doi: 10.1080/02656736.2021.1905189. Int J Hyperthermia. 2021. PMID: 33827375 Free PMC article.
-
The histotripsy spectrum: differences and similarities in techniques and instrumentation.Int J Hyperthermia. 2023;40(1):2233720. doi: 10.1080/02656736.2023.2233720. Int J Hyperthermia. 2023. PMID: 37460101 Free PMC article. Review.
-
The penetration effect of HMME-mediated low-frequency and low-intensity ultrasound against the Staphylococcus aureus bacterial biofilm.Eur J Med Res. 2020 Oct 22;25(1):51. doi: 10.1186/s40001-020-00452-z. Eur J Med Res. 2020. PMID: 33092628 Free PMC article.
-
Histotripsy: an innovative approach for minimally invasive tumour and disease treatment.Ann Med Surg (Lond). 2024 Mar 5;86(4):2081-2087. doi: 10.1097/MS9.0000000000001897. eCollection 2024 Apr. Ann Med Surg (Lond). 2024. PMID: 38576932 Free PMC article. Review.
-
Particle-Mediated Histotripsy for the Targeted Treatment of Intraluminal Biofilms in Catheter-Based Medical Devices.BME Front. 2022 Jul 5;2022:9826279. doi: 10.34133/2022/9826279. eCollection 2022. BME Front. 2022. PMID: 37850182 Free PMC article.
References
-
- Cochrane DM, Brown MR, Anwar H, Weller PH, Lam K, and Costerton JW, “Antibody response to Pseudomonas aeruginosa surface protein antigens in a rat model of chronic lung infection,” J Med Microbiol, vol. 27, pp. 255–261, 1988 1988. - PubMed
-
- De Beer D, Stoodley P, and Lewandowski Z, “Measurement of local diffusion coefficients in biofilms by microinjection and confocal microscopy,” Biotechnology and Bioengineering, vol. 53, pp. 151–158, 1997. - PubMed
-
- Rodgers J, Phillips F, and Olliff C, “The effects of extracellular slime from Staphylococcus epidermidis on phagocytic ingestion and killing,”FEMS Immunology and Medical Microbiology, vol. 9, pp. 109–115, 1994. - PubMed
-
- Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR,DeLeo FR, et al., “Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system,” Cellular Microbiology, vol. 6, pp. 269–275, 2004. - PubMed

