Ubiquilin 2 modulates ALS/FTD-linked FUS-RNA complex dynamics and stress granule formation
- PMID: 30442662
- PMCID: PMC6298105
- DOI: 10.1073/pnas.1811997115
Ubiquilin 2 modulates ALS/FTD-linked FUS-RNA complex dynamics and stress granule formation
Abstract
The ubiquitin-like protein ubiquilin 2 (UBQLN2) has been genetically and pathologically linked to the neurodegenerative diseases amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), but its normal cellular functions are not well understood. In a search for UBQLN2-interacting proteins, we found an enrichment of stress granule (SG) components, including ALS/FTD-linked heterogeneous ribonucleoprotein fused in sarcoma (FUS). Through the use of an optimized SG detection method, we observed UBQLN2 and its interactors at SGs. A low complexity, Sti1-like repeat region in UBQLN2 was sufficient for its localization to SGs. Functionally, UBQLN2 negatively regulated SG formation. UBQLN2 increased the dynamics of FUS-RNA interaction and promoted the fluidity of FUS-RNA complexes at a single-molecule level. This solubilizing effect corresponded to a dispersal of FUS liquid droplets in vitro and a suppression of FUS SG formation in cells. ALS-linked mutations in UBQLN2 reduced its association with FUS and impaired its function in regulating FUS-RNA complex dynamics and SG formation. These results reveal a previously unrecognized role for UBQLN2 in regulating the early stages of liquid-liquid phase separation by directly modulating the fluidity of protein-RNA complexes and the dynamics of SG formation.
Keywords: ALS; FTD; FUS; stress granule; ubiquilin 2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

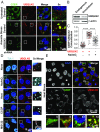
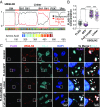
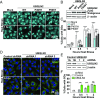


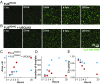
References
-
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. - PubMed
-
- van den Berg-Vos RM, et al. Sporadic lower motor neuron disease with adult onset: Classification of subtypes. Brain. 2003;126:1036–1047. - PubMed
-
- Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010;9:995–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

