Permanent neuroglial remodeling of the retina following infiltration of CSF1R inhibition-resistant peripheral monocytes
- PMID: 30442669
- PMCID: PMC6275537
- DOI: 10.1073/pnas.1807123115
Permanent neuroglial remodeling of the retina following infiltration of CSF1R inhibition-resistant peripheral monocytes
Abstract
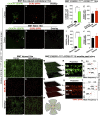
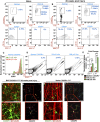
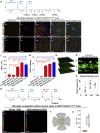
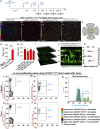
Previous studies have demonstrated that ocular injury can lead to prompt infiltration of bone-marrow-derived peripheral monocytes into the retina. However, the ability of these cells to integrate into the tissue and become microglia has not been investigated. Here we show that such peripheral monocytes that infiltrate into the retina after ocular injury engraft permanently, migrate to the three distinct microglia strata, and adopt a microglia-like morphology. In the absence of ocular injury, peripheral monocytes that repopulate the retina after depletion with colony-stimulating factor 1 receptor (CSF1R) inhibitor remain sensitive to CSF1R inhibition and can be redepleted. Strikingly, consequent to ocular injury, the engrafted peripheral monocytes are resistant to depletion by CSF1R inhibitor and likely express low CSF1R. Moreover, these engrafted monocytes remain proinflammatory, expressing high levels of MHC-II, IL-1β, and TNF-α over the long term. The observed permanent neuroglia remodeling after injury constitutes a major immunological change that may contribute to progressive retinal degeneration. These findings may also be relevant to other degenerative conditions of the retina and the central nervous system.
Keywords: PLX5622; glaucoma; macrophage; neurodegeneration; neuroprotection.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages.Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23336-23338. doi: 10.1073/pnas.1922788117. Epub 2020 Sep 8. Proc Natl Acad Sci U S A. 2020. PMID: 32900927 Free PMC article.
-
Microglia Regulate Neuroglia Remodeling in Various Ocular and Retinal Injuries.J Immunol. 2019 Jan 15;202(2):539-549. doi: 10.4049/jimmunol.1800982. Epub 2018 Dec 12. J Immunol. 2019. PMID: 30541880 Free PMC article.
-
Glaucoma after Ocular Surgery or Trauma: The Role of Infiltrating Monocytes and Their Response to Cytokine Inhibitors.Am J Pathol. 2020 Oct;190(10):2056-2066. doi: 10.1016/j.ajpath.2020.07.006. Epub 2020 Jul 18. Am J Pathol. 2020. PMID: 32693061 Free PMC article.
-
Depletion of embryonic microglia using the CSF1R inhibitor PLX5622 has adverse sex-specific effects on mice, including accelerated weight gain, hyperactivity and anxiolytic-like behaviour.Brain Behav Immun. 2018 Oct;73:682-697. doi: 10.1016/j.bbi.2018.07.023. Epub 2018 Jul 27. Brain Behav Immun. 2018. PMID: 30056204
-
To Kill a Microglia: A Case for CSF1R Inhibitors.Trends Immunol. 2020 Sep;41(9):771-784. doi: 10.1016/j.it.2020.07.001. Epub 2020 Aug 10. Trends Immunol. 2020. PMID: 32792173 Free PMC article. Review.
Cited by
-
Subventricular zone/white matter microglia reconstitute the empty adult microglial niche in a dynamic wave.Elife. 2021 Aug 23;10:e66738. doi: 10.7554/eLife.66738. Elife. 2021. PMID: 34423781 Free PMC article.
-
The CSF1R-Microglia Axis Has Protective Host-Specific Roles During Neurotropic Picornavirus Infection.Front Immunol. 2021 Sep 9;12:621090. doi: 10.3389/fimmu.2021.621090. eCollection 2021. Front Immunol. 2021. PMID: 34566948 Free PMC article.
-
Inflammatory and Fibrogenic Factors in Proliferative Vitreoretinopathy Development.Transl Vis Sci Technol. 2020 Feb 21;9(3):23. doi: 10.1167/tvst.9.3.23. Transl Vis Sci Technol. 2020. PMID: 32742753 Free PMC article. Review.
-
CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages.Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23336-23338. doi: 10.1073/pnas.1922788117. Epub 2020 Sep 8. Proc Natl Acad Sci U S A. 2020. PMID: 32900927 Free PMC article.
-
Microglia Regulate Neuroglia Remodeling in Various Ocular and Retinal Injuries.J Immunol. 2019 Jan 15;202(2):539-549. doi: 10.4049/jimmunol.1800982. Epub 2018 Dec 12. J Immunol. 2019. PMID: 30541880 Free PMC article.
References
-
- Cade F, Grosskreutz CL, Tauber A, Dohlman CH. Glaucoma in eyes with severe chemical burn, before and after keratoprosthesis. Cornea. 2011;30:1322–1327. - PubMed
-
- Crnej A, et al. Glaucoma progression and role of glaucoma surgery in patients with Boston keratoprosthesis. Cornea. 2014;33:349–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

