3-Chlorodiphenylamine activates cardiac troponin by a mechanism distinct from bepridil or TFP
- PMID: 30442775
- PMCID: PMC6314390
- DOI: 10.1085/jgp.201812131
3-Chlorodiphenylamine activates cardiac troponin by a mechanism distinct from bepridil or TFP
Abstract
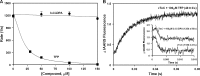
Despite extensive efforts spanning multiple decades, the development of highly effective Ca2+ sensitizers for the heart remains an elusive goal. Existing Ca2+ sensitizers have other targets in addition to cardiac troponin (cTn), which can lead to adverse side effects, such as hypotension or arrhythmias. Thus, there is a need to design Ca2+-sensitizing drugs with higher affinity and selectivity for cTn. Previously, we determined that many compounds based on diphenylamine (DPA) were able to bind to a cTnC-cTnI chimera with moderate affinity (Kd ∼10-120 µM). Of these compounds, 3-chlorodiphenylamine (3-Cl-DPA) bound most tightly (Kd of 10 µM). Here, we investigate 3-Cl-DPA further and find that it increases the Ca2+ sensitivity of force development in skinned cardiac muscle. Using NMR, we show that, like the known Ca2+ sensitizers, trifluoperazine (TFP) and bepridil, 3-Cl-DPA is able to bind to the isolated N-terminal domain (N-domain) of cTnC (Kd of 6 µM). However, while the bulky molecules of TFP and bepridil stabilize the open state of the N-domain of cTnC, the small and flexible 3-Cl-DPA molecule is able to bind without stabilizing this open state. Thus, unlike TFP, which drastically slows the rate of Ca2+ dissociation from the N-domain of isolated cTnC in a dose-dependent manner, 3-Cl-DPA has no effect on the rate of Ca2+ dissociation. On the other hand, the affinity of 3-Cl-DPA for a cTnC-TnI chimera is at least an order of magnitude higher than that of TFP or bepridil, likely because 3-Cl-DPA is less disruptive of cTnI binding to cTnC. Therefore, 3-Cl-DPA has a bigger effect on the rate of Ca2+ dissociation from the entire cTn complex than TFP and bepridil. Our data suggest that 3-Cl-DPA activates the cTn complex via a unique mechanism and could be a suitable scaffold for the development of novel treatments for systolic heart failure.
© 2018 Tikunova et al.
Figures




References
-
- Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . 2018. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 137:e67–e492. 10.1161/CIR.0000000000000558 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

