Heterobiaryl synthesis by contractive C-C coupling via P(V) intermediates
- PMID: 30442804
- PMCID: PMC6814017
- DOI: 10.1126/science.aas8961
Heterobiaryl synthesis by contractive C-C coupling via P(V) intermediates
Abstract
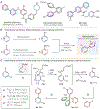
Heterobiaryls composed of pyridine and diazine rings are key components of pharmaceuticals and are often central to pharmacological function. We present an alternative approach to metal-catalyzed cross-coupling to make heterobiaryls using contractive phosphorus C-C couplings, also termed phosphorus ligand coupling reactions. The process starts by regioselective phosphorus substitution of the C-H bonds para to nitrogen in two successive heterocycles; ligand coupling is then triggered via acidic alcohol solutions to form the heterobiaryl bond. Mechanistic studies imply that ligand coupling is an asynchronous process involving migration of one heterocycle to the ipso position of the other around a central pentacoordinate P(V) atom. The strategy can be applied to complex drug-like molecules containing multiple reactive sites and polar functional groups, and also enables convergent coupling of drug fragments and late-stage heteroarylation of pharmaceuticals.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
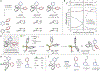
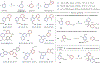

References
-
- Brown DG, Boström J, J. Med. Chem 59, 4443–4458 (2016). - PubMed
-
- Roughley SD, Jordan AM, J. Med. Chem 54, 3451–3479 (2011). - PubMed
-
- Capdeville R, Buchdunger E, Zimmermann J, Matter A, Nat. Rev. Drug Discov 1, 493–502 (2002). - PubMed
-
- Roecker AJ et al. , ChemMedChem 9, 311–322 (2014). - PubMed
-
- Martina SD, Vesta KS, Ripley TL, Ann. Pharmacother 39, 854–862 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

