Long noncoding RNA AC003092.1 promotes temozolomide chemosensitivity through miR-195/TFPI-2 signaling modulation in glioblastoma
- PMID: 30442884
- PMCID: PMC6237774
- DOI: 10.1038/s41419-018-1183-8
Long noncoding RNA AC003092.1 promotes temozolomide chemosensitivity through miR-195/TFPI-2 signaling modulation in glioblastoma
Abstract
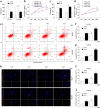
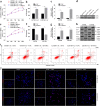
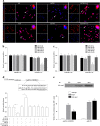
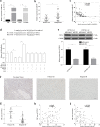
Temozolomide (TMZ) and radiation therapy combination for glioblastoma (GB) patients has been considered as the most effective therapy after surgical procedure. However, the overall clinical prognosis remains unsatisfactory due to intrinsic or developing resistance to TMZ. Recently, increasing evidence suggested that long noncoding RNAs (lncRNAs) play a critical role in various biological processes of tumors, and have been implicated in resistance to various drugs. However, the role of lncRNAs in TMZ resistance is poorly understood. Here, we found that the expression of lncRNA AC003092.1 was markedly decreased in TMZ resistance (TR) of GB cells (U87TR and U251TR) compared with their parental cells (U87 and U251). In patients with glioma, low levels of lncRNA AC003092.1 were correlated with increased TMZ resistance, higher risk of relapse, and poor prognosis. Overexpression of lncRNA AC003092.1 enhances TMZ sensitivity, facilitates cell apoptosis, and inhibits cell proliferation in TMZ-resistant GB cells. In addition, we identified that lncRNA AC003092.1 regulates TMZ chemosensitivity through TFPI-2-mediated cell apoptosis in vitro and in vivo. Mechanistically, further investigation revealed that lncRNA AC003092.1 regulates TFPI-2 expression through miR-195 in GB. Taken together, these data suggest that lncRNA AC003092.1 could inhibit the function of miR-195 by acting as an endogenous CeRNA, leading to increased expression of TFPI-2; this promotes TMZ-induced apoptosis, thereby making GB cells more sensitive to TMZ. Our findings indicate that overexpression of lncRNA AC003092.1 may be a potential therapy to overcome TMZ resistance in GB patients.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Exosomal transfer of long non-coding RNA SBF2-AS1 enhances chemoresistance to temozolomide in glioblastoma.J Exp Clin Cancer Res. 2019 Apr 16;38(1):166. doi: 10.1186/s13046-019-1139-6. J Exp Clin Cancer Res. 2019. PMID: 30992025 Free PMC article.
-
Long noncoding RNA RP11-838N2.4 enhances the cytotoxic effects of temozolomide by inhibiting the functions of miR-10a in glioblastoma cell lines.Oncotarget. 2016 Jul 12;7(28):43835-43851. doi: 10.18632/oncotarget.9699. Oncotarget. 2016. PMID: 27270310 Free PMC article.
-
LncRNA SOX2OT promotes temozolomide resistance by elevating SOX2 expression via ALKBH5-mediated epigenetic regulation in glioblastoma.Cell Death Dis. 2020 May 21;11(5):384. doi: 10.1038/s41419-020-2540-y. Cell Death Dis. 2020. PMID: 32439916 Free PMC article.
-
Drug-induced modifications and modulations of microRNAs and long non-coding RNAs for future therapy against Glioblastoma Multiforme.Gene. 2020 Jan 10;723:144126. doi: 10.1016/j.gene.2019.144126. Epub 2019 Oct 4. Gene. 2020. PMID: 31589963 Review.
-
The Role of Long Noncoding Ribonucleic Acids in Glioblastoma: What the Neurosurgeon Should Know.Neurosurgery. 2023 Jun 1;92(6):1104-1111. doi: 10.1227/neu.0000000000002449. Epub 2023 Mar 1. Neurosurgery. 2023. PMID: 36880757 Review.
Cited by
-
Identification of an immune-related eRNA prognostic signature for clear cell renal cell carcinoma.Aging (Albany NY). 2024 Jan 29;16(3):2232-2248. doi: 10.18632/aging.205479. Epub 2024 Jan 29. Aging (Albany NY). 2024. PMID: 38289619 Free PMC article.
-
Current understanding of functional peptides encoded by lncRNA in cancer.Cancer Cell Int. 2024 Jul 19;24(1):252. doi: 10.1186/s12935-024-03446-7. Cancer Cell Int. 2024. PMID: 39030557 Free PMC article. Review.
-
Comparative analyses of long non-coding RNA profiles in vivo in cystic fibrosis lung airway and parenchyma tissues.Respir Res. 2019 Dec 16;20(1):284. doi: 10.1186/s12931-019-1259-8. Respir Res. 2019. PMID: 31842871 Free PMC article.
-
Role of Non-coding RNAs in the Response of Glioblastoma to Temozolomide.Mol Neurobiol. 2025 Feb;62(2):1726-1755. doi: 10.1007/s12035-024-04316-z. Epub 2024 Jul 18. Mol Neurobiol. 2025. PMID: 39023794 Review.
-
NKILA, a prognostic indicator, inhibits tumor metastasis by suppressing NF-κB/Slug mediated epithelial-mesenchymal transition in hepatocellular carcinoma.Int J Biol Sci. 2020 Jan 1;16(3):495-503. doi: 10.7150/ijbs.39582. eCollection 2020. Int J Biol Sci. 2020. PMID: 32015685 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

