Androgen induced cellular proliferation, neurogenesis, and generation of GnRH3 neurons in the brain of mature female Mozambique tilapia
- PMID: 30442908
- PMCID: PMC6237963
- DOI: 10.1038/s41598-018-35303-9
Androgen induced cellular proliferation, neurogenesis, and generation of GnRH3 neurons in the brain of mature female Mozambique tilapia
Abstract
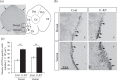
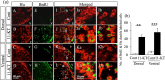
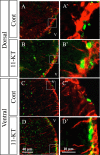
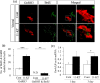
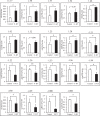
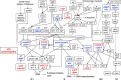
The neuroplastic mechanisms in the fish brain that underlie sex reversal remain unknown. Gonadotropin-releasing hormone 3 (GnRH3) neurons control male reproductive behaviours in Mozambique tilapia and show sexual dimorphism, with males having a greater number of GnRH3 neurons. Treatment with androgens such as 11-ketotestosterone (KT), but not 17β-estradiol, increases the number of GnRH3 neurons in mature females to a level similar to that observed in mature males. Compared with oestrogen, the effect of androgen on neurogenesis remains less clear. The present study examined the effects of 11-KT, a non-aromatizable androgen, on cellular proliferation, neurogenesis, generation of GnRH3 neurons and expression of cell cycle-related genes in mature females. The number of proliferating cell nuclear antigen-positive cells was increased by 11-KT. Simultaneous injection of bromodeoxyuridine and 11-KT significantly increased the number of newly-generated (newly-proliferated) neurons, but did not affect radial glial cells, and also resulted in newly-generated GnRH3 neurons. Transcriptome analysis showed that 11-KT modulates the expression of genes related to the cell cycle process. These findings suggest that tilapia could serve as a good animal model to elucidate the effects of androgen on adult neurogenesis and the mechanisms for sex reversal in the fish brain.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Pandian TJ, Sheela SG. Hormonal induction of sex reversal in fish. Aquaculture. 1995;138:1–22. doi: 10.1016/0044-8486(95)01075-0. - DOI
-
- Phelps, R. P. & Popma, T. J. Sex reversal of tilapia. Tilapia aquaculture in the Americas, Vol. 2. The World Aquaculture Society, 34–59 (2000).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

