Developmental induction of human T-cell responses against Candida albicans and Aspergillus fumigatus
- PMID: 30442915
- PMCID: PMC6238003
- DOI: 10.1038/s41598-018-35161-5
Developmental induction of human T-cell responses against Candida albicans and Aspergillus fumigatus
Abstract
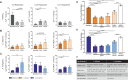
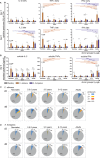
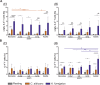
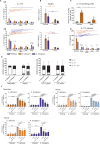
The origin of human T-cell responses against fungal pathogens early in life is not clearly understood. Here, we show that antifungal T-cell responses are vigorously initiated within the first years of life against lysates and peptides of Candida albicans or Aspergillus fumigatus, presented by autologous monocytes. The neonatal responding T-cell pool consists of 20 different TCR-Vβ families, whereas infant and adult pools display dramatically less variability. Although we demonstrate no bias for anti-fungal IL-4 expression early in life, there was a strong bias for anti-fungal IL-17 production. Of note, only T-cells from neonates and infants show an immediate co-expression of multiple cytokines. In addition, only their T-cells co-express simultaneously transcription factors T-bet and RORγt in response to fungi and subsequently their target genes IL-17 and IFNγ. Thus, T-cells of neonates and infants are predetermined to respond quickly with high plasticity to fungal pathogens, which might give an excellent opportunity for therapeutic interventions.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Cross-protective TH1 immunity against Aspergillus fumigatus and Candida albicans.Blood. 2011 Jun 2;117(22):5881-91. doi: 10.1182/blood-2010-12-325084. Epub 2011 Mar 25. Blood. 2011. PMID: 21441461
-
Pulmonary immune responses against Aspergillus fumigatus are characterized by high frequencies of IL-17 producing T-cells.J Infect. 2017 Jan;74(1):81-88. doi: 10.1016/j.jinf.2016.10.010. Epub 2016 Nov 9. J Infect. 2017. PMID: 27838522
-
Human dendritic cells following Aspergillus fumigatus infection express the CCR7 receptor and a differential pattern of interleukin-12 (IL-12), IL-23, and IL-27 cytokines, which lead to a Th1 response.Infect Immun. 2006 Mar;74(3):1480-9. doi: 10.1128/IAI.74.3.1480-1489.2006. Infect Immun. 2006. PMID: 16495518 Free PMC article.
-
Dendritic cells and fungi.APMIS. 2003 Jul-Aug;111(7-8):789-96. doi: 10.1034/j.1600-0463.2003.11107810.x. APMIS. 2003. PMID: 12974780 Review.
-
Immune responses to Aspergillus fumigatus infections.Biol Blood Marrow Transplant. 2006 Jan;12(1 Suppl 1):47-9. doi: 10.1016/j.bbmt.2005.09.007. Biol Blood Marrow Transplant. 2006. PMID: 16399584 Review.
Cited by
-
Immune-checkpoint blockade of CTLA-4 (CD152) in antigen-specific human T-cell responses differs profoundly between neonates, children, and adults.Oncoimmunology. 2021 Jun 14;10(1):1938475. doi: 10.1080/2162402X.2021.1938475. Oncoimmunology. 2021. PMID: 34178430 Free PMC article.
-
Frequency of synaptic antigen-specific CD4+ T cells in dementia is age-dependent but not correlated with cognitive impairment.Immun Ageing. 2025 Jun 19;22(1):23. doi: 10.1186/s12979-025-00516-w. Immun Ageing. 2025. PMID: 40537813 Free PMC article.
-
The accuracy of the Candida Score® in predicting the likelihood of fungal sepsis in newborns.Ann Med. 2025 Dec;57(1):2548022. doi: 10.1080/07853890.2025.2548022. Epub 2025 Aug 22. Ann Med. 2025. PMID: 40844406 Free PMC article.
-
Insights into the Role of Commensal-Specific T Cells in Intestinal Inflammation.J Inflamm Res. 2022 Mar 14;15:1873-1887. doi: 10.2147/JIR.S288288. eCollection 2022. J Inflamm Res. 2022. PMID: 35342295 Free PMC article. Review.
-
Development of a Simple and Robust Whole Blood Assay with Dual Co-Stimulation to Quantify the Release of T-Cellular Signature Cytokines in Response to Aspergillus fumigatus Antigens.J Fungi (Basel). 2021 Jun 8;7(6):462. doi: 10.3390/jof7060462. J Fungi (Basel). 2021. PMID: 34201183 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

