Identification of genetic variants associated with tacrolimus metabolism in kidney transplant recipients by extreme phenotype sampling and next generation sequencing
- PMID: 30442921
- PMCID: PMC6522337
- DOI: 10.1038/s41397-018-0063-z
Identification of genetic variants associated with tacrolimus metabolism in kidney transplant recipients by extreme phenotype sampling and next generation sequencing
Abstract
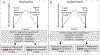
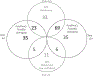
An extreme phenotype sampling (EPS) model with targeted next-generation sequencing (NGS) identified genetic variants associated with tacrolimus (Tac) metabolism in subjects from the Deterioration of Kidney Allograft Function (DeKAF) Genomics cohort which included 1,442 European Americans (EA) and 345 African Americans (AA). This study included 48 subjects separated into 4 groups of 12 (AA high, AA low, EA high, EA low). Groups were selected by the extreme phenotype of dose-normalized Tac trough concentrations after adjusting for common genetic variants and clinical factors. NGS spanned > 3 Mb of 28 genes and identified 18,661 genetic variants (3961 previously unknown). A group of 125 deleterious variants, by SIFT analysis, were associated with Tac troughs in EAs (burden test, p = 0.008), CYB5R2 was associated with Tac troughs in AAs (SKAT, p = 0.00079). In CYB5R2, rs61733057 (increased allele frequency in AAs) was predicted to disrupt protein function by SIFT and PolyPhen2 analysis. The variants merit further validation.
Conflict of interest statement
Conflict of Interest
The authors declare no conflicts of interest for this study.
Figures

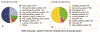
References
-
- Annual Data Report of the US Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR). Preface. Am J Transplant 2013; 13 Suppl 1: 1–7. - PubMed
-
- Endrenyi L, Tothfalusi L. Determination of bioequivalence for drugs with narrow therapeutic index: reduction of the regulatory burden. J Pharm Pharm Sci 2013; 16(5): 676–682. - PubMed
-
- Gaynor JJ, Ciancio G, Guerra G, Sageshima J, Roth D, Goldstein MJ, et al. Lower tacrolimus trough levels are associated with subsequently higher acute rejection risk during the first 12 months after kidney transplantation. Transpl Int 2016; 29(2): 216–226. - PubMed
-
- Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation 1996; 62(7): 900–905. - PubMed
-
- Lancia P, Jacqz-Aigrain E, Zhao W. Choosing the right dose of tacrolimus. Arch Dis Child 2015; 100(4): 406–413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

