Revised diagnostic criteria for plasma cell leukemia: results of a Mayo Clinic study with comparison of outcomes to multiple myeloma
- PMID: 30442928
- PMCID: PMC6238010
- DOI: 10.1038/s41408-018-0140-1
Revised diagnostic criteria for plasma cell leukemia: results of a Mayo Clinic study with comparison of outcomes to multiple myeloma
Abstract
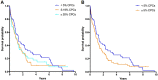
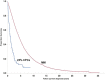
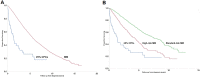
The current definition of plasma cell leukemia (PCL)- ≥ 20% circulating plasma cells (CPCs) on peripheral smear and plasma cell count ≥ 2 × 109/L-may be too stringent. We reviewed outcomes of 176 multiple myeloma (MM) patients diagnosed between 1971 and 2016, and who had CPCs detectable at diagnosis, to determine whether a lower threshold could be used to diagnose PCL. Median overall survival (mOS) was 1.1 years (95% CI 0.8-1.4) and was similar between patients with < 5% (n = 54, mOS = 1.4 years [0.7-2.0]), 5-19% (n = 63, mOS = 1.1 years [0.7-1.4]), and ≥ 20% CPCs (n = 59, mOS = 1.1 years [0.7-1.5], p = 0.349). As survival was similar between those with 5-19% and ≥ 20% CPCs, we stratified patients by < 5% (mOS = 1.4 years [0.7-2.0]) and ≥ 5% CPCs (mOS = 1.1 years [0.8-1.4], p = 0.154). Outcomes of those with ≥ 5% CPCs were much poorer when compared with a cohort of MM patients diagnosed between 1971 and 2016, who did not have CPCs at diagnosis (n = 9724, mOS = 4.4 yrs [4.3-4.5], p < 0.001); survival was also lower in patients diagnosed after 2001 with ≥ 5% CPCs (n = 62, mOS = 1.4 years [0.8-2.5]) compared with patients with standard risk (n = 1326, mOS = 7.5 years [7.0-8.7]) and high-risk MM (n = 381, mOS = 4.3 years [3.5-4.9], p < 0.001). We therefore propose that the definition of PCL be revised to patients with ≥ 5% CPCs on peripheral blood smear, who otherwise meet diagnostic criteria for MM.
Conflict of interest statement
S.K. has obtained research support for clinical trials from Celgene, Millennium, Novartis, Janssen, and Sanofi. A.D. has received research support for clinical trials from Pfizer, Jannsen, Millennium, Alnylam, and Celgene. M.A.G. has received research support from ISIS and Prothena, and honoraria from Celgene, Millennium Pharmaceuticals, and Novartis. The remaining authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

