Dysregulation of autophagy and stress granule-related proteins in stress-driven Tau pathology
- PMID: 30442948
- PMCID: PMC6748085
- DOI: 10.1038/s41418-018-0217-1
Dysregulation of autophagy and stress granule-related proteins in stress-driven Tau pathology
Abstract
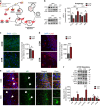
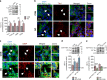
Imbalance of neuronal proteostasis associated with misfolding and aggregation of Tau protein is a common neurodegenerative feature in Alzheimer's disease (AD) and other Tauopathies. Consistent with suggestions that lifetime stress may be an important AD precipitating factor, we previously reported that environmental stress and high glucocorticoid (GC) levels induce accumulation of aggregated Tau; however, the molecular mechanisms for such process remain unclear. Herein, we monitor a novel interplay between RNA-binding proteins (RBPs) and autophagic machinery in the underlying mechanisms through which chronic stress and high GC levels impact on Tau proteostasis precipitating Tau aggregation. Using molecular, pharmacological and behavioral analysis, we demonstrate that chronic stress and high GC trigger mTOR-dependent inhibition of autophagy, leading to accumulation of Tau aggregates and cell death in P301L-Tau expressing mice and cells. In parallel, we found that environmental stress and GC disturb cellular homeostasis and trigger the insoluble accumulation of different RBPs, such as PABP, G3BP1, TIA-1, and FUS, shown to form stress granules (SGs) and Tau aggregation. Interestingly, an mTOR-driven pharmacological stimulation of autophagy attenuates the GC-driven accumulation of Tau and SG-related proteins as well as the related cell death, suggesting a critical interface between autophagy and the response of the SG-related protein in the neurodegenerative potential of chronic stress and GC. These studies provide novel insights into the RNA-protein intracellular signaling regulating the precipitating role of environmental stress and GC on Tau-driven brain pathology.
Conflict of interest statement
B.W. is co-founder and chief scientific officer of Aquinnah Pharmaceutics Inc. The other authors declare that they have no conflict of interest.
Figures






Similar articles
-
Contrasting pathology of the stress granule proteins TIA-1 and G3BP in tauopathies.J Neurosci. 2012 Jun 13;32(24):8270-83. doi: 10.1523/JNEUROSCI.1592-12.2012. J Neurosci. 2012. PMID: 22699908 Free PMC article.
-
Molecular interaction of stress granules with Tau and autophagy in Alzheimer's disease.Neurochem Int. 2022 Jul;157:105342. doi: 10.1016/j.neuint.2022.105342. Epub 2022 Apr 21. Neurochem Int. 2022. PMID: 35461975 Review.
-
Decrease of neuronal FKBP4/FKBP52 modulates perinuclear lysosomal positioning and MAPT/Tau behavior during MAPT/Tau-induced proteotoxic stress.Autophagy. 2021 Nov;17(11):3491-3510. doi: 10.1080/15548627.2021.1875611. Epub 2021 Jan 25. Autophagy. 2021. PMID: 33459145 Free PMC article.
-
mTOR regulates tau phosphorylation and degradation: implications for Alzheimer's disease and other tauopathies.Aging Cell. 2013 Jun;12(3):370-80. doi: 10.1111/acel.12057. Epub 2013 Mar 24. Aging Cell. 2013. PMID: 23425014 Free PMC article.
-
Tau Protein Squired by Molecular Chaperones During Alzheimer's Disease.J Mol Neurosci. 2018 Nov;66(3):356-368. doi: 10.1007/s12031-018-1174-3. Epub 2018 Sep 28. J Mol Neurosci. 2018. PMID: 30267382 Review.
Cited by
-
Photobiomodulation and visual stimulation against cognitive decline and Alzheimer's disease pathology: A systematic review.Alzheimers Dement (N Y). 2022 Nov 25;8(1):e12249. doi: 10.1002/trc2.12249. eCollection 2022. Alzheimers Dement (N Y). 2022. PMID: 36447479 Free PMC article. Review.
-
Stress Granules Modulate SYK to Cause Tau-Associated Neurocognitive Deterioration in 5XFAD Mouse After Anesthesia and Surgery.Front Aging Neurosci. 2021 Aug 27;13:718701. doi: 10.3389/fnagi.2021.718701. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34512311 Free PMC article.
-
Altering biomolecular condensates as a potential mechanism that mediates cannabidiol effect on glioblastoma.Med Oncol. 2024 May 7;41(6):140. doi: 10.1007/s12032-024-02381-x. Med Oncol. 2024. PMID: 38713310
-
Effect of calcium ions on the aggregation of highly phosphorylated tau.Biochem Biophys Rep. 2024 Nov 24;40:101887. doi: 10.1016/j.bbrep.2024.101887. eCollection 2024 Dec. Biochem Biophys Rep. 2024. PMID: 39655264 Free PMC article.
-
Fyn-dependent Tau microcluster formation seeds and boosts extensive Tau pathology.Acta Neuropathol. 2025 May 14;149(1):48. doi: 10.1007/s00401-025-02887-2. Acta Neuropathol. 2025. PMID: 40366450
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

