Amyloid β toxic conformer has dynamic localization in the human inferior parietal cortex in absence of amyloid plaques
- PMID: 30442978
- PMCID: PMC6237870
- DOI: 10.1038/s41598-018-35004-3
Amyloid β toxic conformer has dynamic localization in the human inferior parietal cortex in absence of amyloid plaques
Abstract
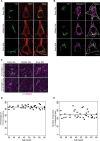
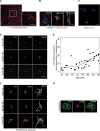
Amyloid β (Aβ) plays a critical role in the pathogenesis of Alzheimer's disease. Nevertheless, its distribution and clearance before Aβ plaque formation needs to be elucidated. Using an optimized immunofluorescent staining method, we examined the distribution of Aβ in the post-mortem parietal cortex of 35 subjects, 30 to 65 years of age, APOE ε3/ε3, without AD lesions. We used 11A1, an antibody against an Aβ conformer which forms neurotoxic oligomers. 11A1 immunoreactivity (IR) was present in cortical neurons, pericapillary spaces, astrocytes and the extracellular compartment at 30 years of age. The percentage of neurons with 11A1 IR did not change with age, but the number and percentage of astrocytes with 11A1 IR gradually increased. Notably, the percentage of pericapillary spaces labeled with 11A1 IR declined significantly in the 5th decade of the life, at the same time that 11A1 IR increased in the extracellular space. Our findings indicate that the Aβ toxic conformer is normally present in various cell types and brain parenchyma, and appears to be constitutively produced, degraded, and cleared from the inferior parietal cortex. The decrease in pericapillary Aβ and the concomitant increase of extracellular Aβ may reflect an age-associated impairment in Aβ clearance from the brain.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Human intravenous immunoglobulin provides protection against Aβ toxicity by multiple mechanisms in a mouse model of Alzheimer's disease.J Neuroinflammation. 2010 Dec 7;7:90. doi: 10.1186/1742-2094-7-90. J Neuroinflammation. 2010. PMID: 21138577 Free PMC article.
-
Non-toxic conformer of amyloid β may suppress amyloid β-induced toxicity in rat primary neurons: implications for a novel therapeutic strategy for Alzheimer's disease.Biochem Biophys Res Commun. 2013 Aug 16;438(1):1-5. doi: 10.1016/j.bbrc.2013.05.106. Epub 2013 Jun 4. Biochem Biophys Res Commun. 2013. PMID: 23747423
-
Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in Swedish mutant APP transgenic mice.Am J Pathol. 2007 Feb;170(2):680-92. doi: 10.2353/ajpath.2007.060378. Am J Pathol. 2007. PMID: 17255335 Free PMC article.
-
[Deposition and clearance of β-amyloid in the brain].Brain Nerve. 2013 Dec;65(12):1433-44. Brain Nerve. 2013. PMID: 24323930 Review. Japanese.
-
[Development of a Novel Alzheimer's Disease Model Based on the Theory of the Toxic-conformer of Amyloid β].Yakugaku Zasshi. 2021;141(6):843-849. doi: 10.1248/yakushi.20-00251-5. Yakugaku Zasshi. 2021. PMID: 34078792 Review. Japanese.
Cited by
-
Jatrorrhizine Balances the Gut Microbiota and Reverses Learning and Memory Deficits in APP/PS1 transgenic mice.Sci Rep. 2019 Dec 20;9(1):19575. doi: 10.1038/s41598-019-56149-9. Sci Rep. 2019. PMID: 31862965 Free PMC article.
-
DNA damage accumulates and responses are engaged in human ALS brain and spinal motor neurons and DNA repair is activatable in iPSC-derived motor neurons with SOD1 mutations.Acta Neuropathol Commun. 2020 Jan 31;8(1):7. doi: 10.1186/s40478-019-0874-4. Acta Neuropathol Commun. 2020. PMID: 32005289 Free PMC article.
-
A brain proteomic signature of incipient Alzheimer's disease in young APOE ε4 carriers identifies novel drug targets.Sci Adv. 2021 Nov 12;7(46):eabi8178. doi: 10.1126/sciadv.abi8178. Epub 2021 Nov 10. Sci Adv. 2021. PMID: 34757788 Free PMC article.
-
Immunohistochemical Study of Mitochondrial Ferritin in the Midbrain of Patients with Progressive Supranuclear Palsy.Acta Histochem Cytochem. 2021 Jun 29;54(3):97-104. doi: 10.1267/ahc.21-00019. Epub 2021 Jun 23. Acta Histochem Cytochem. 2021. PMID: 34276103 Free PMC article.
-
Proteasome localization and activity in pig brain and in vivo small molecule screening for activators.Front Cell Neurosci. 2024 Feb 26;18:1353542. doi: 10.3389/fncel.2024.1353542. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38469354 Free PMC article.
References
-
- Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

