Estrogen-dependent DLL1-mediated Notch signaling promotes luminal breast cancer
- PMID: 30442981
- PMCID: PMC6756232
- DOI: 10.1038/s41388-018-0562-z
Estrogen-dependent DLL1-mediated Notch signaling promotes luminal breast cancer
Abstract
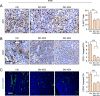
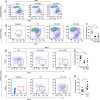
Aberrant Notch signaling is implicated in several cancers, including breast cancer. However, the mechanistic details of the specific receptors and function of ligand-mediated Notch signaling that promote breast cancer remains elusive. In our studies we show that DLL1, a Notch signaling ligand, is significantly overexpressed in ERα+ luminal breast cancer. Intriguingly, DLL1 overexpression correlates with poor prognosis in ERα+ luminal breast cancer, but not in other subtypes of breast cancer. In addition, this effect is specific to DLL1, as other Notch ligands (DLL3, JAGGED1, and JAGGED2) do not influence the clinical outcome of ERα+ patients. Genetic studies show that DLL1-mediated Notch signaling in breast cancer is important for tumor cell proliferation, angiogenesis, and cancer stem cell function. Consistent with prognostic clinical data, we found the tumor-promoting function of DLL1 is exclusive to ERα+ luminal breast cancer, as loss of DLL1 inhibits both tumor growth and lung metastasis of luminal breast cancer. Importantly, we find that estrogen signaling stabilizes DLL1 protein by preventing its proteasomal and lysososmal degradations. Moreover, estrogen inhibits ubiquitination of DLL1. Together, our results highlight an unexpected and novel subtype-specific function of DLL1 in promoting luminal breast cancer that is regulated by estrogen signaling. Our studies also emphasize the critical role of assessing subtype-specific mechanisms driving tumor growth and metastasis to generate effective subtype-specific therapeutics.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells.Oncogene. 2011 Sep 29;30(39):4075-86. doi: 10.1038/onc.2011.122. Epub 2011 Apr 18. Oncogene. 2011. PMID: 21499308 Free PMC article.
-
Production and characterization of a novel Delta-like 1 functional unit as a tool for Notch pathway activation and generation of a specific antibody.Protein Expr Purif. 2018 Jun;146:8-16. doi: 10.1016/j.pep.2018.01.008. Epub 2018 Jan 31. Protein Expr Purif. 2018. PMID: 29366964
-
Maintenance of Bone Homeostasis by DLL1-Mediated Notch Signaling.J Cell Physiol. 2017 Sep;232(9):2569-2580. doi: 10.1002/jcp.25647. Epub 2017 Mar 31. J Cell Physiol. 2017. PMID: 27735989 Free PMC article.
-
Possible roles of DLK1 in the Notch pathway during development and disease.Biochim Biophys Acta. 2012 Jun;1822(6):988-95. doi: 10.1016/j.bbadis.2012.02.003. Epub 2012 Feb 12. Biochim Biophys Acta. 2012. PMID: 22353464 Review.
-
Notch Signaling in Breast Cancer: A Role in Drug Resistance.Cells. 2020 Sep 29;9(10):2204. doi: 10.3390/cells9102204. Cells. 2020. PMID: 33003540 Free PMC article. Review.
Cited by
-
In-Silico Characterization of Estrogen Reactivating β-Glucuronidase Enzyme in GIT Associated Microbiota of Normal Human and Breast Cancer Patients.Genes (Basel). 2022 Aug 27;13(9):1545. doi: 10.3390/genes13091545. Genes (Basel). 2022. PMID: 36140713 Free PMC article.
-
Angioregulatory microRNAs in breast cancer: Molecular mechanistic basis and implications for therapeutic strategies.J Adv Res. 2021 Jun 26;37:235-253. doi: 10.1016/j.jare.2021.06.019. eCollection 2022 Mar. J Adv Res. 2021. PMID: 35499045 Free PMC article. Review.
-
Consequences of EMT-Driven Changes in the Immune Microenvironment of Breast Cancer and Therapeutic Response of Cancer Cells.J Clin Med. 2019 May 9;8(5):642. doi: 10.3390/jcm8050642. J Clin Med. 2019. PMID: 31075939 Free PMC article. Review.
-
Notch signaling in breast cancer: From pathway analysis to therapy.Cancer Lett. 2019 Oct 1;461:123-131. doi: 10.1016/j.canlet.2019.07.012. Epub 2019 Jul 18. Cancer Lett. 2019. PMID: 31326555 Free PMC article. Review.
-
The many facets of Notch signaling in breast cancer: toward overcoming therapeutic resistance.Genes Dev. 2020 Nov 1;34(21-22):1422-1438. doi: 10.1101/gad.342287.120. Genes Dev. 2020. PMID: 33872192 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

