Inhibition of FAO in AML co-cultured with BM adipocytes: mechanisms of survival and chemosensitization to cytarabine
- PMID: 30442990
- PMCID: PMC6237992
- DOI: 10.1038/s41598-018-35198-6
Inhibition of FAO in AML co-cultured with BM adipocytes: mechanisms of survival and chemosensitization to cytarabine
Abstract
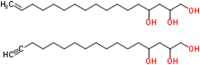
Adipocytes are the prevalent stromal cell type in adult bone marrow (BM), and leukemia cells continuously adapt to deficiency of nutrients acquiring chemoresistant profiles in the BM microenvironment. We have previously shown that fatty acid metabolism is a key energy pathway for survival of acute myeloid leukemia (AML) cells in the adipocyte-abundant BM microenvironment. The novel fatty acid β-oxidation (FAO) inhibitor avocatin B, an odd-numbered carbon lipid derived from the avocado fruit, induced apoptosis and growth inhibition in mono-cultured AML cells. In AML cells co-cultured with BM adipocytes, FAO inhibition with avocatin B caused adaptive stimulation of free fatty acid (FFA) uptake through upregulation of FABP4 mRNA, enhanced glucose uptake and switch to glycolysis. These changes reflect the compensatory response to a shortage of FFA supply to the mitochondria, and facilitate the protection of AML cells from avocatin B-induced apoptosis in the presence of BM adipocytes. However, the combination treatment of avocatin B and conventional anti-AML therapeutic agent cytarabine (AraC) increased reactive oxygen species and demonstrated highly synergistic effects on AML cells under BM adipocyte co-culture condition. These findings highlight the potential for combination regimens of AraC and FAO inhibitors that target bone marrow-resident chemoresistant AML cells.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Fatty Acid Metabolism, Bone Marrow Adipocytes, and AML.Front Oncol. 2020 Feb 18;10:155. doi: 10.3389/fonc.2020.00155. eCollection 2020. Front Oncol. 2020. PMID: 32133293 Free PMC article. Review.
-
Bone Marrow Adipocytes Facilitate Fatty Acid Oxidation Activating AMPK and a Transcriptional Network Supporting Survival of Acute Monocytic Leukemia Cells.Cancer Res. 2017 Mar 15;77(6):1453-1464. doi: 10.1158/0008-5472.CAN-16-1645. Epub 2017 Jan 20. Cancer Res. 2017. PMID: 28108519 Free PMC article.
-
TGF-β-Neutralizing Antibody 1D11 Enhances Cytarabine-Induced Apoptosis in AML Cells in the Bone Marrow Microenvironment.PLoS One. 2013 Jun 27;8(6):e62785. doi: 10.1371/journal.pone.0062785. Print 2013. PLoS One. 2013. PMID: 23826077 Free PMC article.
-
Consolidation Chemotherapy Prevents Relapse by Indirectly Regulating Bone Marrow Adipogenesis in Patients with Acute Myeloid Leukemia.Cell Physiol Biochem. 2018;45(6):2389-2400. doi: 10.1159/000488225. Epub 2018 Mar 13. Cell Physiol Biochem. 2018. PMID: 29550830
-
[Energy metabolism of acute myeloid leukemia cells in the bone marrow microenvironment].Rinsho Ketsueki. 2022;63(9):1046-1051. doi: 10.11406/rinketsu.63.1046. Rinsho Ketsueki. 2022. PMID: 36198528 Review. Japanese.
Cited by
-
Mitochondrial metabolism as a target for acute myeloid leukemia treatment.Cancer Metab. 2021 Apr 21;9(1):17. doi: 10.1186/s40170-021-00253-w. Cancer Metab. 2021. PMID: 33883040 Free PMC article. Review.
-
Targeting the NRF2/HO-1 Antioxidant Pathway in FLT3-ITD-Positive AML Enhances Therapy Efficacy.Antioxidants (Basel). 2022 Apr 5;11(4):717. doi: 10.3390/antiox11040717. Antioxidants (Basel). 2022. PMID: 35453402 Free PMC article.
-
mTOR Regulation of Metabolism in Hematologic Malignancies.Cells. 2020 Feb 11;9(2):404. doi: 10.3390/cells9020404. Cells. 2020. PMID: 32053876 Free PMC article. Review.
-
Turning the spotlight on bone marrow adipocytes in haematological malignancy and non-malignant conditions.Br J Haematol. 2023 May;201(4):605-619. doi: 10.1111/bjh.18748. Epub 2023 Apr 17. Br J Haematol. 2023. PMID: 37067783 Free PMC article. Review.
-
Fatty Acid Metabolism, Bone Marrow Adipocytes, and AML.Front Oncol. 2020 Feb 18;10:155. doi: 10.3389/fonc.2020.00155. eCollection 2020. Front Oncol. 2020. PMID: 32133293 Free PMC article. Review.
References
-
- Farge Thomas, Saland Estelle, de Toni Fabienne, Aroua Nesrine, Hosseini Mohsen, Perry Robin, Bosc Claudie, Sugita Mayumi, Stuani Lucille, Fraisse Marine, Scotland Sarah, Larrue Clément, Boutzen Héléna, Féliu Virginie, Nicolau-Travers Marie-Laure, Cassant-Sourdy Stéphanie, Broin Nicolas, David Marion, Serhan Nizar, Sarry Audrey, Tavitian Suzanne, Kaoma Tony, Vallar Laurent, Iacovoni Jason, Linares Laetitia K., Montersino Camille, Castellano Rémy, Griessinger Emmanuel, Collette Yves, Duchamp Olivier, Barreira Yara, Hirsch Pierre, Palama Tony, Gales Lara, Delhommeau François, Garmy-Susini Barbara H., Portais Jean-Charles, Vergez François, Selak Mary, Danet-Desnoyers Gwenn, Carroll Martin, Récher Christian, Sarry Jean-Emmanuel. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discovery. 2017;7(7):716–735. doi: 10.1158/2159-8290.CD-16-0441. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

