Telomerase gene expression bioassays indicate metabolic activation of genotoxic lower chlorinated polychlorinated biphenyls
- PMID: 30443001
- PMCID: PMC6237825
- DOI: 10.1038/s41598-018-35043-w
Telomerase gene expression bioassays indicate metabolic activation of genotoxic lower chlorinated polychlorinated biphenyls
Abstract
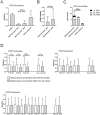
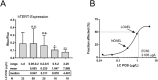
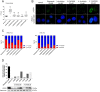
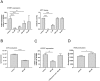
Polychlorinated biphenyls (PCBs) are ubiquitously occurring pollutants with different chemical and toxicological properties. In this study we evaluated blood plasma samples of two PCB-exposed cohorts for their ability to alter telomerase (hTERT) gene expression. Blood plasma from PCB-exposed individuals inhibited hTERT expression depending solely on the concentration of lower chlorinated PCBs, with the lowest observed adverse effect level (LOAEL) at a plasma concentration between 0.5 and 2 µg/L of LC PCBs. Individual OH-metabolites derived from the WHO indicator congeners PCB 28 and PCB 101 mimicked these effects on hTERT expression in vitro with high toxicity, including DNA damage. However, by the combination of different OH-metabolites, the bio effective PCB concentration was reduced and the respective effects on hTERT expression could be increased. At a concentration which showed no toxic activity in MTT assay, hTERT inhibition reflected the interference of OH-PCBs with the mitochondrial respiratory chain, which could lead to the production of reactive oxygen species (ROS). As individual OH-metabolites already showed a much stronger inhibition of hTERT gene expression at a lower concentration than their parental compounds, the hTERT gene expression bioassay described in this study seems to indicate metabolic activation of LC PCBs rather than the mere effect of LC PCBs on their own. In summary, this study provides dose-response linkages between effects of lower chlorinated PCBs and their concentrations in human plasma.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

