The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases
- PMID: 30443043
- PMCID: PMC6535143
- DOI: 10.1038/s41574-018-0115-0
The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases
Abstract
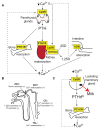
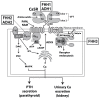
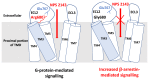
The Ca2+-sensing receptor (CaSR) is a dimeric family C G protein-coupled receptor that is expressed in calcitropic tissues such as the parathyroid glands and the kidneys and signals via G proteins and β-arrestin. The CaSR has a pivotal role in bone and mineral metabolism, as it regulates parathyroid hormone secretion, urinary Ca2+ excretion, skeletal development and lactation. The importance of the CaSR for these calcitropic processes is highlighted by loss-of-function and gain-of-function CaSR mutations that cause familial hypocalciuric hypercalcaemia and autosomal dominant hypocalcaemia, respectively, and also by the fact that alterations in parathyroid CaSR expression contribute to the pathogenesis of primary and secondary hyperparathyroidism. Moreover, the CaSR is an established therapeutic target for hyperparathyroid disorders. The CaSR is also expressed in organs not involved in Ca2+ homeostasis: it has noncalcitropic roles in lung and neuronal development, vascular tone, gastrointestinal nutrient sensing, wound healing and secretion of insulin and enteroendocrine hormones. Furthermore, the abnormal expression or function of the CaSR is implicated in cardiovascular and neurological diseases, as well as in asthma, and the CaSR is reported to protect against colorectal cancer and neuroblastoma but increase the malignant potential of prostate and breast cancers.
Conflict of interest statement
F.M.H and R.V.T. have received grant funding from NPS/Shire Pharmaceuticals and GlaxoSmithKline for studies involving the use of calcium-sensing receptor allosteric inhibitors.
Figures
References
-
- Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. - PubMed
-
- Ward DT, Brown EM, Harris HW. Disulfide bonds in the extracellular calcium-polyvalent cation-sensing receptor correlate with dimer formation and its response to divalent cations in vitro. J Biol Chem. 1998;273:14476–14483. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

