Plasmodium falciparum Treated with Artemisinin-based Combined Therapy Exhibits Enhanced Mutation, Heightened Cortisol and TNF-α Induction
- PMID: 30443164
- PMCID: PMC6216064
- DOI: 10.7150/ijms.27350
Plasmodium falciparum Treated with Artemisinin-based Combined Therapy Exhibits Enhanced Mutation, Heightened Cortisol and TNF-α Induction
Abstract
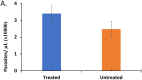
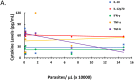
The artemisinin-based combined therapy (ACT) post-treatment illness in Plasmodium falciparum-endemic areas is characterized by vague malaria-like symptoms. The roles of treatment modality, persistence of parasites and host proinflammatory response in disease course are unknown. We investigated the hypothesis that ACT post-treatment syndrome is driven by parasite genetic polymorphisms and proinflammatory response to persisting mutant parasites. Patients were categorized as treated, untreated and malaria-negative. Malaria positive samples were analyzed for Pfcrt, Pfmdr1, K13 kelch gene polymorphisms, while all samples were evaluated for cytokines (TNF-α, IL-12p70, IL-10, TGF-β, IFN-γ) and corticosteroids (cortisol and dexamethasone) levels. The treated patients exhibited higher levels of parasitemia, TNF-α, and cortisol, increased incidence of parasite genetic mutations, and greater number of mutant alleles per patient. In addition, corticosteroid levels declined with increasing number of mutant alleles. TGF-β levels were negatively correlated with parasitemia, while IL-10 and TGF-β were negatively correlated with increasing number of mutant alleles. However, IL-12 displayed slight positive correlation and TNF-α exhibited moderate positive correlation with increasing number of mutant alleles. Since post-treatment management ultimately results in patient recovery, the high parasite gene polymorphism may act in concert with induced cortisol and TNF-α to account for ACT post-treatment syndrome.
Keywords: ACT.; Proinflammation; corticosteroids; cortisol; dexamethasone; gene polymorphism; persistent malaria.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures








References
-
- Rogier C, Trape J, Commenges D. Evidence for an Age-Dependent Pyrogenic Threshold of Plasmodium falciparum Parasitemia in Highly Endemic Populations. Am J Trop Med Hyg. 1996;54:613–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

