Inhibition of AKT suppresses the initiation and progression of BRCA1-associated mammary tumors
- PMID: 30443181
- PMCID: PMC6231214
- DOI: 10.7150/ijbs.29242
Inhibition of AKT suppresses the initiation and progression of BRCA1-associated mammary tumors
Abstract
Despite the high incidence of BRCA1-mutant breast cancer, few substantial improvements in preventing or treating such cancers have been made. Using a Brca1-mutant mouse model, we examined the contribution of AKT to the incidence and growth of Brca1-mutated mammary tumors. A haploinsufficiency of Akt1 in Brca1-mutant mouse model significantly decreased mammary tumor formation from 54% in Brca1co/coMMTV-Cre mice to 22% in Brca1 co/coMMTV-Cre Akt1+/- mice. Notably, treatment of tumor-bearing Brca1-mutant mice with the AKT-inhibitor, MK-2206, yielded partial response or stable disease up to 91% of mice in maximum response. MK-2206 treatment also significantly reduced tumor volume and delayed recurrence in allograft and adjuvant studies, respectively. A correlation analysis of MK-2206 responses with gene expression profiles of tumors at baseline identified seven genes that were differentially expressed between tumors that did and did not respond to MK-2206 treatment. Our findings enhance our understanding of the involvement of AKT signaling in BRCA1-deficient mammary tumors and provide preclinical evidence that targeted AKT inhibition is a potential strategy for the prevention and therapeutic management of BRCA1-associated breast cancer.
Keywords: AKT; BRCA1; MK-2206; precision medicine.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



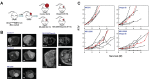

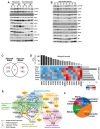
References
-
- Caestecker KW, Van de Walle GR. The role of BRCA1 in DNA double-strand repair: past and present. Exp Cell Res. 2013;319:575–87. - PubMed
-
- Takaoka M, Miki Y. BRCA1 gene: function and deficiency. Int J Clin Oncol. 2018;23:36–44. - PubMed
-
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

