Hypoxia Inducible Factor as a Central Regulator of Metabolism - Implications for the Development of Obesity
- PMID: 30443205
- PMCID: PMC6221908
- DOI: 10.3389/fnins.2018.00813
Hypoxia Inducible Factor as a Central Regulator of Metabolism - Implications for the Development of Obesity
Abstract
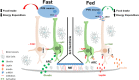
The hypothalamus plays a major role in the regulation of food intake and energy expenditure. In the last decade, it was demonstrated that consumption of high-fat diets triggers the activation of an inflammatory process in the hypothalamus, inducing neurofunctional alterations and contributing to the development of obesity. Hypoxia-inducible factors (HIFs) are key molecules that regulate cellular responses to inflammation and hypoxia, being essential for the normal cell function and survival. Currently, evidence points to a role of HIF pathway in metabolic regulation that could also be involved in the progression of obesity and metabolic diseases. The challenge is to understand how HIF modulation impacts body mass gain and metabolic disorders such as insulin resistance. Distinct animal models with tissue-specific knocking-out or overexpression of hypoxia signaling pathway genes revealed a cell-specificity in the activation of HIF pathways, and some of them have opposite phenotypes among the various HIFs gain- and loss-of-function mouse models. In this review, we discuss the major findings that provide support for a role of HIF pathway involvement in the regulation of metabolism, especially in glucose and energy homeostasis.
Keywords: HIF complex; energy homeostasis; hypothalamus; inflammation; metabolic disorders; obesity.
Figures


References
-
- Belgardt B. F., Husch A., Rother E., Ernst M. B., Wunderlich F. T., Hampel B., et al. (2008). PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 7 291–301. 10.1016/j.cmet.2008.01.006 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

