Manipulation of Salmonella Typhi Gene Expression Impacts Innate Cell Responses in the Human Intestinal Mucosa
- PMID: 30443257
- PMCID: PMC6221971
- DOI: 10.3389/fimmu.2018.02543
Manipulation of Salmonella Typhi Gene Expression Impacts Innate Cell Responses in the Human Intestinal Mucosa
Abstract
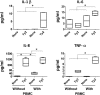
Although immunity induced by typhoid fever is moderated and short-lived, typhoid vaccination with the attenuated Ty21a oral vaccine generates long-lasting protection rates reaching up to 92%. Thus, there are important differences on how wild-type Salmonella and typhoid vaccine strains stimulate host immunity. We hypothesize that vaccine strains with different mutations might affect gut inflammation and intestinal permeability by different mechanisms. To test this hypothesis, we used an in vitro organotypic model of the human intestinal mucosa composed of human intestinal epithelial cells, lymphocytes/monocytes, endothelial cells, and fibroblasts. We also used six Salmonella enterica serovar Typhi (S. Typhi) strains: the licensed Ty21a oral vaccine, four typhoid vaccine candidates (i.e., CVD 908, CVD 909, CVD 910, and CVD 915) and the wild-type Ty2 strain. We found that genetically engineered S. Typhi vaccine strains elicit differential host changes not only in the intestinal permeability and secretion of inflammatory cytokines, but also in the phenotype and activation pathways of innate cells. These changes were distinct from those elicited by the parent wild-type S. Typhi and depended on the genetic manipulation. In sum, these results emphasize the importance of carefully selecting specific manipulations of the Salmonella genome in the development of typhoid vaccines.
Keywords: Salmonella; gut; human; innate immunity; mucosal immunity; typhoid vaccine.
Figures










References
-
- Levine MM. Typhoid fever vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plokin's Vaccines. Philadelphia, PA: Elsevier, Inc; (2018). 1114–44.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

