The hallmarks of living systems: towards creating artificial cells
- PMID: 30443324
- PMCID: PMC6227776
- DOI: 10.1098/rsfs.2018.0023
The hallmarks of living systems: towards creating artificial cells
Abstract
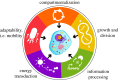
Despite the astonishing diversity and complexity of living systems, they all share five common hallmarks: compartmentalization, growth and division, information processing, energy transduction and adaptability. In this review, we give not only examples of how cells satisfy these requirements for life and the ways in which it is possible to emulate these characteristics in engineered platforms, but also the gaps that remain to be bridged. The bottom-up synthesis of life-like systems continues to be driven forward by the advent of new technologies, by the discovery of biological phenomena through their transplantation to experimentally simpler constructs and by providing insights into one of the oldest questions posed by mankind, the origin of life on Earth.
Keywords: adaptability; artificial cell; compartmentalization; division; energy transduction; information processing.
Conflict of interest statement
We have no competing interests.
Figures




References
-
- Mason AF, Thordarson P. 2017. Polymersomes as protocellular constructs. J. Polym. Sci. Part A Polym. Chem. 55, 3817–3825. (10.1002/pola.28780) - DOI
Publication types
LinkOut - more resources
Full Text Sources

