Onset timing of statin-induced musculoskeletal adverse events and concomitant drug-associated shift in onset timing of MAEs
- PMID: 30443347
- PMCID: PMC6220123
- DOI: 10.1002/prp2.439
Onset timing of statin-induced musculoskeletal adverse events and concomitant drug-associated shift in onset timing of MAEs
Abstract
To evaluate the onset timing of musculoskeletal adverse events (MAEs) that develop during statin monotherapy and to determine whether concomitant drugs used concurrently with statin therapy shifts the onset timing of MAEs. Cases in which statins (atorvastatin, rosuvastatin, simvastatin, lovastatin, fluvastatin, pitavastatin, and pravastatin) were prescribed were extracted from the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) Data Files. The onset timing of MAEs during statin monotherapy was evaluated by determining the difference between statin start date and MAE onset date. The use of concomitant drugs with statin therapy was included in the analysis. Statins used in combination with concomitant drugs were compared with statin monotherapy to determine if the use of concomitant drugs shifted the onset timing of MAEs. The onset of MAEs was significantly faster with atorvastatin and rosuvastatin than with simvastatin. A difference in onset timing was not detected with other statins because the number of cases was too small for analysis. When evaluating concomitant drug use, the concomitant drugs that shifted the onset timing of MAEs could not be detected. Statins with strong low-density lipoprotein cholesterol-lowering effects (atorvastatin and rosuvastatin) contributed not only to a high risk of MAE onset, but also to a shorter time-to-onset. No concomitant drug significantly shifted the onset timing of MAEs when used concurrently with statins.
Keywords: concomitant drug; drug‐drug interaction; musculoskeletal adverse event; onset timing; rhabdomyolysis; statin.
Figures
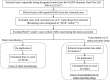
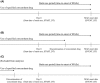
References
-
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1‐S45. - PubMed
-
- Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781‐1790. - PubMed
-
- Chang JT, Staffa JA, Parks M, et al. Rhabdomyolysis with HMG‐CoA reductase inhibitors and gemfibrozil combination therapy. Pharmacoepidemiol Drug Saf. 2004;13:417‐426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

