Crystal structure of the thalidomide analog (3a R*,7a S*)-2-(2,6-dioxopiperidin-3-yl)hexa-hydro-1 H-iso-indole-1,3(2 H)-dione
- PMID: 30443388
- PMCID: PMC6218906
- DOI: 10.1107/S2056989018014317
Crystal structure of the thalidomide analog (3a R*,7a S*)-2-(2,6-dioxopiperidin-3-yl)hexa-hydro-1 H-iso-indole-1,3(2 H)-dione
Abstract
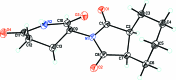
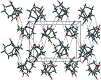
The title compound, C13H16N2O4, crystallizes in the monoclinic centrosymmetric space group, P21/c, with four mol-ecules in the asymmetric unit, thus there is no crystallographically imposed symmetry and it is a racemic mixture. The structure consists of a six-membered unsaturated ring bound to a five-membered pyrrolidine-2,5-dione ring N-bound to a six-membered piperidine-2,6-dione ring and thus has the same basic skeleton as thalidomide, except for the six-membered unsaturated ring substituted for the aromatic ring. In the crystal, the mol-ecules are linked into inversion dimers by R 2 2(8) hydrogen bonding involving the N-H group. In addition, there are bifurcated C-H⋯O inter-actions involving one of the O atoms on the pyrrolidine-2,5-dione with graph-set notation R 1 2(5). These inter-actions along with C-H⋯O inter-actions involving one of the O atoms on the piperidine-2,6-dione ring link the mol-ecules into a complex three-dimensional array. There is pseudomerohedral twinning present which results from a 180° rotation about the [100] reciprocal lattice direction and with a twin law of 1 0 0 0 0 0 0 [BASF 0.044 (1)].
Keywords: crystal structure; pseudomerohedral twinning; thalidomide analogs.
Figures
References
-
- Allen, F. H. & Trotter, J. (1971). J. Chem. Soc. B, pp. 1073–1079.
-
- Bartlett, J. B., Dredge, K. & Dalgleish, A. G. (2004). Nat. Rev. Cancer, 4, 314–322. - PubMed
-
- Begna, K., Pardanani, A., Mesa, R., Litzow, M., Hogan, W., Hanson, C. & Tefferi, A. (2012). Am. J. Hematol. 87, 66–68. - PubMed
-
- Benjamin, E. & Hijji, Y. M. (2017). J. Chem. pp. 1–6.
-
- Blaschke, G., Kraft, H. P., Fickentscher, K. & Köhler, F. (1979). Arzneim.-Forsch. 29, 1640–1642. - PubMed
LinkOut - more resources
Full Text Sources
