Evaluation of intraductal delivery of poly(ethylene glycol)-doxorubicin conjugate nanocarriers for the treatment of ductal carcinoma in situ (DCIS)-like lesions in rats
- PMID: 30443411
- PMCID: PMC6220801
- DOI: 10.1002/jin2.51
Evaluation of intraductal delivery of poly(ethylene glycol)-doxorubicin conjugate nanocarriers for the treatment of ductal carcinoma in situ (DCIS)-like lesions in rats
Abstract
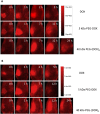
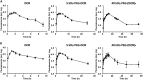
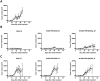
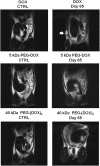
Ductal carcinoma in situ is the most commonly diagnosed early stage breast cancer. The efficacy of intraductally delivered poly(ethylene glycol)-doxorubicin (PEG-DOX) nanocarriers, composed of one or more DOX conjugated to various PEG polymers, was investigated in an orthotopic ductal carcinoma in situ-like rat model. In vitro cytotoxicity was evaluated against 13762 Mat B III cells using MTT assay. The orthotopic model was developed by inoculating cancer cells into mammary ducts of female Fischer 344 retired breeder rats. The ductal retention and in vivo antitumour efficacy of two of the six nanocarriers (5 kDa PEG-DOX and 40 kDa PEG-(DOX)4) were investigated based on in vitro results. Mammary retention of DOX and PEG-DOX nanocarriers was quantified using in vivo imaging. Histopathologic effects of DOX and PEG-DOX nanocarriers on mammary ductal structure were also investigated. Cytotoxicities of small linear PEG-DOX nanocarriers (5 and 10 kDa) were not different from DOX whereas larger PEG-DOX nanocarriers showed reduced potency. The order of mammary retention was 40 kDa PEG-(DOX)4 > 5 kDa PEG-DOX >> DOX, in normal and tumour-bearing rats. Intraductally administered PEG-DOX nanocarriers and DOX were effective in reducing tumour incidence and increasing survival rate, with no significant differences found among the three treatment groups. However, nanocarriers administered intravenously at the same doses were not effective, and intraductally administered free DOX caused severe local toxicity. Intraductal administration of PEG-DOX nanocarriers is effective and less toxic than that of free DOX, as well as IV DOX/PEG-DOX. Furthermore, PEG-DOX nanocarriers demonstrate the added benefit of prolonging DOX ductal retention, which would necessitate less frequent dosing.
Keywords: DCIS rat model; PEG nanocarrier; doxorubicin; ductal carcinoma in situ (DCIS); intraductal therapy; mammary gland retention.
Figures




Similar articles
-
The effect of size and polymer architecture of doxorubicin-poly(ethylene) glycol conjugate nanocarriers on breast duct retention, potency and toxicity.Eur J Pharm Sci. 2018 Aug 30;121:118-125. doi: 10.1016/j.ejps.2018.04.033. Epub 2018 Apr 24. Eur J Pharm Sci. 2018. PMID: 29698706
-
Breast intraductal nanoformulations for treating ductal carcinoma in situ I: Exploring metal-ion complexation to slow ciclopirox release, enhance mammary persistence and efficacy.J Control Release. 2020 Jul 10;323:71-82. doi: 10.1016/j.jconrel.2020.04.016. Epub 2020 Apr 14. J Control Release. 2020. PMID: 32302762
-
Multi-functional nanocarriers based on iron oxide nanoparticles conjugated with doxorubicin, poly(ethylene glycol) and folic acid as theranostics for cancer therapy.Colloids Surf B Biointerfaces. 2018 Oct 1;170:529-537. doi: 10.1016/j.colsurfb.2018.06.051. Epub 2018 Jun 26. Colloids Surf B Biointerfaces. 2018. PMID: 29966906
-
Intraductal delivery of nanocarriers for ductal carcinoma in situ treatment: a strategy to enhance localized delivery.Nanomedicine (Lond). 2022 Oct;17(24):1871-1889. doi: 10.2217/nnm-2022-0234. Epub 2023 Jan 25. Nanomedicine (Lond). 2022. PMID: 36695306 Review.
-
Legumain-cleavable 4-arm poly(ethylene glycol)-doxorubicin conjugate for tumor specific delivery and release.Acta Biomater. 2017 May;54:227-238. doi: 10.1016/j.actbio.2017.03.019. Epub 2017 Mar 15. Acta Biomater. 2017. PMID: 28315495
Cited by
-
Non-systemic Approaches for Ductal Carcinoma In Situ: Exploring the Potential of Ultra-flexible Combisomes as a Novel Drug Delivery Strategy-a Review.AAPS PharmSciTech. 2023 May 12;24(5):119. doi: 10.1208/s12249-023-02574-z. AAPS PharmSciTech. 2023. PMID: 37173545 Review.
-
Collagen-Binding Nanoparticles for Paclitaxel Encapsulation and Breast Cancer Treatment.ACS Biomater Sci Eng. 2023 Dec 11;9(12):6805-6820. doi: 10.1021/acsbiomaterials.3c01332. Epub 2023 Nov 20. ACS Biomater Sci Eng. 2023. PMID: 37982792 Free PMC article.
-
The INTEND 1 randomized controlled trial of duct endoscopy as an indicator of margin excision in breast conservation surgery.Breast Cancer Res Treat. 2021 Apr;186(3):723-730. doi: 10.1007/s10549-020-06065-8. Epub 2021 Jan 4. Breast Cancer Res Treat. 2021. PMID: 33392842 Clinical Trial.
-
Breast intraductal nanoformulations for treating ductal carcinoma in situ II: Dose de-escalation using a slow releasing/slow bioconverting prodrug strategy.Drug Deliv Transl Res. 2022 Jan;12(1):240-256. doi: 10.1007/s13346-021-00903-y. Epub 2021 Feb 15. Drug Deliv Transl Res. 2022. PMID: 33590464
References
-
- Burstein, H. J. , Polyak, K. , Wong, J. S. , Lester, S. C. , and Kaelin, C. M. 2004. Ductal carcinoma in situ of the breast. N. Engl. J. Med. 350:1430–1441. - PubMed
-
- Chen, Z. , Mei, D. , Dai, Y.‐Y. , and Tang, Z.‐Q. 2010. Plasma concentration of doxorubicin after intraductal administration of doxorubicin long‐circulating liposome in patients with breast cancer. Chinese Pharm J 45:1585–1588.
Grants and funding
LinkOut - more resources
Full Text Sources
