Magnetic Resonance Imaging Volumetry of Primary Nasopharyngeal Cancer in Patients Treated with Induction Gemcitabine and Cisplatin Followed by Concurrent Cisplatin and Volumetric Modulated Arc Therapy
- PMID: 30443466
- PMCID: PMC6235650
- DOI: 10.7759/cureus.3296
Magnetic Resonance Imaging Volumetry of Primary Nasopharyngeal Cancer in Patients Treated with Induction Gemcitabine and Cisplatin Followed by Concurrent Cisplatin and Volumetric Modulated Arc Therapy
Abstract
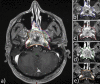
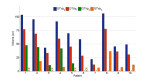
Introduction The addition of induction chemotherapy (IC) to the standard concurrent chemoradiotherapy (CCRT) is under consideration in locally advanced nasopharyngeal carcinoma (LANPC). To-date, no studies have reported primary gross tumour volume (GTVp) changes using gemcitabine and cisplatin as the IC phase in LANPC. We investigated the timing and magnitude of GTVp response throughout sequential gemcitabine and cisplatin IC and CCRT for LANPC. Toxicity and tumour control probability (TCP) analyses are also presented Methods Ten patients with LANPC underwent sequential IC and CCRT between 2011 and 2015. All patients had magnetic resonance imaging (MRI) at three time points: before IC (MRI0), after IC (MRI1), and three months after CCRT (MRI3). Five of the 10 patients had an additional MRI four to five weeks into CCRT (MRI2). GTVp contours were delineated retrospectively using contrast-enhanced MRIs, and each GTVp underwent secondary review by a neuroradiologist. Acute toxicities were graded retrospectively via chart review based on the National Cancer Institute Common Terminology for Adverse Events version 4.0 (NCI CTCAE v4.0). Results Mean GTVp reduction between MRI0 - MRI1 was from 68 cc to 47 cc and from 47 cc to 9 cc between MRI1 - MRI3. In patients with MRI2, the mean GTVp reduction between MRI1 - MRI2 was from 57 cc to 32 cc. Tumour control probability estimates increased by 0.11 after IC. Patients tolerated the treatment well with one Grade IV toxicity event. Conclusion The observed GTVp response and improved tumor control probability support further investigation into the use of IC in LANPC.
Keywords: concurrent chemoradiation; induction chemotherapy; magnetic resonance imagining (mri); nasopharyngeal carcinoma.
Conflict of interest statement
The authors have declared financial relationships, which are detailed in the next section.
Figures

References
-
- The American Joint Committee on Cancer: the 7th edition of the AJCC Cancer Staging Manual and the future of TNM. Edge SB, Compton CC. Ann Surg Oncol. 2010;17:1471–1474. - PubMed
-
- The seventh edition of the UICC/AJCC Staging System for Nasopharyngeal Carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Chen L, Mao Y, Xie F, et al. Radiother Oncol. 2012;104:331–337. - PubMed
-
- Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. Wee J, Tan EH, Tai BC, et al. J Clin Oncol. 2005;23:6730–6738. - PubMed
LinkOut - more resources
Full Text Sources
