Membrane Hsp70-A Novel Target for the Isolation of Circulating Tumor Cells After Epithelial-to-Mesenchymal Transition
- PMID: 30443493
- PMCID: PMC6223102
- DOI: 10.3389/fonc.2018.00497
Membrane Hsp70-A Novel Target for the Isolation of Circulating Tumor Cells After Epithelial-to-Mesenchymal Transition
Abstract
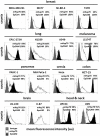
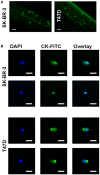
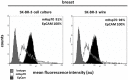
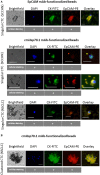
The presence of circulating tumor cells (CTCs) in the peripheral blood is a pre-requisite for progression, invasion, and metastatic spread of cancer. Consequently, the enumeration and molecular characterization of CTCs from the peripheral blood of patients with solid tumors before, during and after treatment serves as a valuable tool for categorizing disease, evaluating prognosis and for predicting and monitoring therapeutic responsiveness. Many of the techniques for isolating CTCs are based on the expression of epithelial cell surface adhesion molecule (EpCAM, CD326) on tumor cells. However, the transition of adherent epithelial cells to migratory mesenchymal cells (epithelial-to-mesenchymal transition, EMT)-an essential element of the metastatic process-is frequently associated with a loss of expression of epithelial cell markers, including EpCAM. A highly relevant proportion of mesenchymal CTCs cannot therefore be isolated using techniques that are based on the "capture" of cells expressing EpCAM. Herein, we provide evidence that a monoclonal antibody (mAb) directed against a membrane-bound form of Hsp70 (mHsp70)-cmHsp70.1-can be used for the isolation of viable CTCs from peripheral blood of tumor patients of different entities in a more quantitative manner. In contrast to EpCAM, the expression of mHsp70 remains stably upregulated on migratory, mesenchymal CTCs, metastases and cells that have been triggered to undergo EMT. Therefore, we propose that approaches for isolating CTCs based on the capture of cells that express mHsp70 using the cmHsp70.1 mAb are superior to those based on EpCAM expression.
Keywords: EpCAM (CD326); circulating tumor cells (CTCs); cmHsp70.1 antibody; epithelial-to-mesenchymal transition (EMT); membrane Hsp70.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

