A guide to maximizing the therapeutic potential of protein-polymer conjugates by rational design
- PMID: 30443654
- PMCID: PMC6322549
- DOI: 10.1039/c8cs00606g
A guide to maximizing the therapeutic potential of protein-polymer conjugates by rational design
Abstract
Proteins are an important class of therapeutics that have advantages including high target specificity, but challenges to their use include rapid clearance and low physical stability. Conjugation of synthetic polymers is an effective approach to address the drawbacks and enhance other properties such as solubility. In this review, we present various considerations in synthesizing protein-polymer conjugates for therapeutic applications with a focus on the choice of polymer, protein, and conjugation method, as well as characterization and evaluation of the resulting conjugate in order to maximize the therapeutic potential of the protein drug.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures
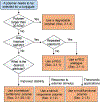

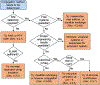
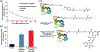
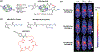
References
-
- Leader B, Baca QJ and Golan DE, Nat. Rev. Drug Discovery, 2008, 7, 21–39. - PubMed
-
- Samanen J, Introduction to Biological and Small Molecule Drug Research and Development, Elsevier, 2013, pp. 161–203.
-
- McGrath NA, Brichacek M and Njardarson JT, J. Chem. Educ, 2010, 87, 1348–1349.
-
- Duncan R, Nat. Rev. Drug Discovery, 2003, 2, 347–360. - PubMed
-
- Pelegri-O’Day EM, Lin E-W and Maynard HD, J. Am. Chem. Soc, 2014, 136, 14323–14332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

