Are aromatase inhibitors associated with higher myocardial infarction risk in breast cancer patients? A Medicare population-based study
- PMID: 30443921
- PMCID: PMC6344290
- DOI: 10.1002/clc.23114
Are aromatase inhibitors associated with higher myocardial infarction risk in breast cancer patients? A Medicare population-based study
Abstract
Background: Theoretically, the estrogen deprivation induced by aromatase inhibitors (AIs) might cause ischemic heart disease, but empiric studies have shown mixed results. We aimed to compare AIs and tamoxifen with regard to cardiovascular events among older breast cancer patients outside of clinical trials. We hypothesized that AIs increase the risk of myocardial infarction.
Methods: We identified women age ≥67 years diagnosed with breast cancer from June 30, 2006 to June 1, 2008 in the surveillance, epidemiology, and end results (SEER)-Medicare database, treated with either tamoxifen or an AI, and followed through December 31, 2012. To compare myocardial infarction (MI) risk for the treatment groups of AIs vs tamoxifen, we developed and assigned stabilized probability of treatment weights and used the Fine and Gray model for time to MI with death not related to MI as a competing risk.
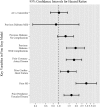
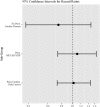
Results: Of the cohort of 5648 women, 4690 were treated with AIs and 958 with tamoxifen; a total of 251 patients developed MI, and 22 patients died of MI during the study period while 476 died of other causes. The hazard for MI was not significantly different between AI vs tamoxifen groups (HR = 1.01, 95% CI 0.72-1.42), after adjusting for the following known MI risk factors at the start of adjuvant therapy: diabetes, ischemic heart disease, congestive heart failure, MI, and peripheral vascular disease.
Conclusions: In this SEER-Medicare-based population study, there were no significant differences in the risk of MI between AI and tamoxifen users after adjustment for known risk factors.
Keywords: adjuvant hormonal therapy; and cardiotoxicity; aromatase inhibitors; breast cancer; tamoxifen.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Author contributions
Sailaja Kamaraju is contributed in conceptualization, coordination of methodology, data collection, writing the original draft, and editing/review and writing of the final manuscript. Yushu Shi is contributed in data collection, formal analysis, writing the original manuscript, reviewing the final manuscript. Elizabeth Smith is contributed in methodology, and statistical analysis, reviewing the final draft. Ann B. Nattinger is contributed in conceptualization, and review and editing of the final manuscript. Purushottam Laud is contributed in design of methodology, statistical analysis, data analysis, review and editing of the final manuscript. Joan Neuner is contributed in conceptualization, data collection, methodology, an overview of the statistical analysis, overall supervision, and writing ‐review, and editing of the manuscript.
Figures
Similar articles
-
The risk of myocardial infarction with aromatase inhibitors relative to tamoxifen in post-menopausal women with early stage breast cancer.Eur J Cancer. 2016 Nov;68:11-21. doi: 10.1016/j.ejca.2016.08.022. Epub 2016 Sep 30. Eur J Cancer. 2016. PMID: 27693889
-
Fractures in a nationwide population-based cohort of users of breast cancer hormonal therapy.J Cancer Surviv. 2018 Apr;12(2):268-275. doi: 10.1007/s11764-017-0666-4. Epub 2017 Dec 15. J Cancer Surviv. 2018. PMID: 29243101
-
Risk of myocardial infarction, stroke, and fracture in a cohort of community-based breast cancer patients.Breast Cancer Res Treat. 2012 Jan;131(2):589-97. doi: 10.1007/s10549-011-1754-1. Epub 2011 Sep 1. Breast Cancer Res Treat. 2012. PMID: 21881937
-
Incidence and management of side effects associated with aromatase inhibitors in the adjuvant treatment of breast cancer in postmenopausal women.Curr Med Res Opin. 2006 Aug;22(8):1609-21. doi: 10.1185/030079906X115667. Curr Med Res Opin. 2006. PMID: 16870085 Review.
-
Safety profiles of tamoxifen and the aromatase inhibitors in adjuvant therapy of hormone-responsive early breast cancer.Ann Oncol. 2007 Sep;18 Suppl 8:viii26-35. doi: 10.1093/annonc/mdm263. Ann Oncol. 2007. PMID: 17890211 Review.
Cited by
-
Association Between Aromatase Inhibitors and Myocardial Infarction Morbidity in Women With Breast Cancer: A Meta-Analysis of Observational Studies.Cancer Control. 2022 Jan-Dec;29:10732748221132512. doi: 10.1177/10732748221132512. Cancer Control. 2022. PMID: 36346929 Free PMC article.
-
Risk of Cardiovascular Disease in Women With and Without Breast Cancer: The Pathways Heart Study.J Clin Oncol. 2022 May 20;40(15):1647-1658. doi: 10.1200/JCO.21.01736. Epub 2022 Apr 6. J Clin Oncol. 2022. PMID: 35385342 Free PMC article.
-
Cardiotoxicity of Use of Sequential Aromatase Inhibitors in Women With Breast Cancer.Am J Epidemiol. 2020 Oct 1;189(10):1086-1095. doi: 10.1093/aje/kwaa065. Am J Epidemiol. 2020. PMID: 32338279 Free PMC article.
-
Duration of aromatase inhibitor use and long-term cardiovascular risk in breast cancer survivors.JNCI Cancer Spectr. 2025 Jan 3;9(1):pkaf009. doi: 10.1093/jncics/pkaf009. JNCI Cancer Spectr. 2025. PMID: 39873699 Free PMC article.
-
Effects of tamoxifen and aromatase inhibitors on the risk of acute coronary syndrome in elderly breast cancer patients: An analysis of nationwide data.Breast. 2020 Dec;54:25-30. doi: 10.1016/j.breast.2020.08.003. Epub 2020 Aug 19. Breast. 2020. PMID: 32890789 Free PMC article.
References
-
- Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine‐responsive early breast cancer: Update of study BIG 1–98. J Clin Oncol. 2007;25(5):486‐492. JCO.2006.08.8617. - PubMed
-
- Boccardo F, Guglielmini P, Rubagotti A, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: long‐term results of the Italian tamoxifen anastrozole (ITA) trial. European J Cancer. 2013;49(7):1546‐1554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical